Journal of Environmental Chemical Engineering ( IF 7.7 ) Pub Date : 2020-08-02 , DOI: 10.1016/j.jece.2020.104316 Asmaa N.A. Hosain , Ahmed El Nemr , Amany El Sikaily , Mohamed E. Mahmoud , Mohamed F. Amira
|
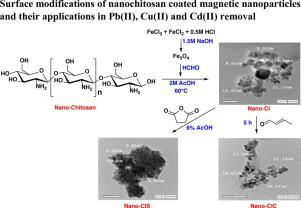
Nano-chitosan coating nano-iron oxide was prepared and its surface was modified with two different compounds (crotonaldehyde and succinic anhydride). The prepared nano-materials (Nano-CI, nano-CIC and nano-CIS) were characterized by FTIR, TGA, XRD, SEM, TEM and BET analyses. Scanning electron microscope (SEM) and transmission electron microscope (TEM) were exhibit average size between 9.32–20.57 nm for nano-materials. Nano-CI, nano-CIC and nano-CIS sorbents were studied for adsorption of Pb(II), Cu(II) and Cd(II). The adsorption process of heavy metal ions was investigated by batch equilibrium technique and several controlling factors were studied. The maximum adsorption capacity values were distinguished in the following order Cu(II) > Pb(II) > Cd(II) as 4700, 2700, and 1800 µmol g–1, respectively. The adsorption equilibrium time was found at (10−30) min for studied heavy metal ions. The applicability of nano-CI, nano-CIC and nano-CIS sorbents to absorb toxic heavy metal ions from sea water and wastewater samples was tested by using micro-column technique. The effect of adsorption temperature (25, 35, 45 and 55 ± 1 °C) on the adsorption of heavy metals by nano-CI, nano-CIC and nano-CIS adsorbents was also investigated. The pseudo-second order is the best and more fitted for the adsorption reactions. Langmuir is the best fitted for removal of the studied metals.
中文翻译:

纳米壳聚糖包覆的磁性纳米粒子的表面修饰及其在去除Pb(II),Cu(II)和Cd(II)中的应用
制备了纳米壳聚糖涂料纳米氧化铁,并用两种不同的化合物(巴豆醛和琥珀酸酐)对其表面进行了改性。通过FTIR,TGA,XRD,SEM,TEM和BET分析对制备的纳米材料(Nano-CI,nano-CIC和nano-CIS)进行了表征。扫描电子显微镜(SEM)和透射电子显微镜(TEM)的纳米材料平均尺寸在9.32–20.57 nm之间。研究了纳米CI,纳米CIC和纳米CIS吸附剂对Pb(II),Cu(II)和Cd(II)的吸附。采用间歇平衡技术研究了重金属离子的吸附过程,并研究了几种控制因素。最大吸附容量值按以下顺序区分:Cu(II)> Pb(II)> Cd(II)为4700、2700和1800 µmol g –1, 分别。研究的重金属离子的吸附平衡时间为(10-30)min。采用微柱技术测试了纳米CI,纳米CIC和纳米CIS吸附剂对海水和废水样品中有毒重金属离子的吸收能力。还研究了吸附温度(25、35、45和55±1°C)对纳米CI,纳米CIC和纳米CIS吸附剂吸附重金属的影响。准二级是最好的,更适合吸附反应。Langmuir最适合去除所研究的金属。



























 京公网安备 11010802027423号
京公网安备 11010802027423号