当前位置:
X-MOL 学术
›
J. Mol. Struct.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Effect of alkyl substituent on molecular configuration in a Cu(II) complex: Synthesis of Cu and CuO nanoparticles using a single, solid-source precursor
Journal of Molecular Structure ( IF 3.8 ) Pub Date : 2021-01-01 , DOI: 10.1016/j.molstruc.2020.129011 Pitchai Selvam , Subramani Sathiyakumar , Krishnan Srinivasan , Thathan Premkumar
Journal of Molecular Structure ( IF 3.8 ) Pub Date : 2021-01-01 , DOI: 10.1016/j.molstruc.2020.129011 Pitchai Selvam , Subramani Sathiyakumar , Krishnan Srinivasan , Thathan Premkumar
|
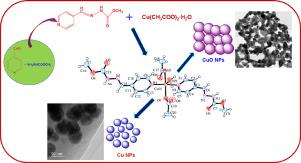
Abstract A new hydrazone-type ligand, methyl 4-(pyridine-4yl-methylene) hydrazinecarboxylate (C8H9N3O2; 4-pymc) and its Cu(II) complex [Cu(4-pymc)2(CH3COO)2(H2O)] (1) were prepared and characterized. The structure of 1 comprises monomers in which Cu is five-coordinated with a distorted square pyramidal structure. Oxygen atoms of two monodentate acetate anions and a pair of nitrogen atoms from pyridine rings form the basal plane. The apical position is filled by a water molecule. Further, the TEM images demonstrated successful preparation of both Cu (~35 nm) and CuO (~95 nm) nanoparticles (NPs) using the single source as-prepared Cu(II) complex via a thermal decomposition approach simply by changing the atmospheric conditions (i.e., vacuum and air atmospheres) under which the Cu(II) complex was calcined for 1 h at 400 °C. Furthermore, the TG-DTA studies indicate that 1 underwent endo- followed by exo-thermic decomposition to form CuO as the end residue in an air atmosphere. The production of Cu and CuO NPs was corroborated by powder x-ray diffraction (PXRD) analysis. The sharp and high intensity PXRD peaks of the calcined samples corroborated that the formed NPs had no impurity and high crystallinity. Furthermore, both ligand and Cu(II) complex showed photoluminescence properties. Also, we demonstrated that the prepared 1 can act as a single, solid-source precursor to form both Cu and CuO NPs.
中文翻译:

烷基取代基对 Cu(II) 复合物中分子构型的影响:使用单一固体源前驱体合成 Cu 和 CuO 纳米颗粒
摘要 一种新的腙型配体4-(吡啶-4基-亚甲基)肼羧酸甲酯(C8H9N3O2; 4-pymc)及其Cu(II)配合物[Cu(4-pymc)2(CH3COO)2(H2O)]( 1) 制备和表征。1的结构包括单体,其中Cu是五配位的,具有扭曲的四方锥体结构。两个单齿乙酸根阴离子的氧原子和来自吡啶环的一对氮原子形成基面。顶端位置被水分子填充。此外,TEM 图像证明使用单源制备的 Cu(II) 复合物通过热分解方法成功制备了 Cu (~35 nm) 和 CuO (~95 nm) 纳米粒子 (NPs),只需改变大气条件(即真空和空气气氛),在此条件下,Cu(II) 配合物在 400 °C 下煅烧 1 小时。此外,TG-DTA 研究表明 1 经历了内分解和放热分解,在空气中形成了作为最终残留物的 CuO。通过粉末 X 射线衍射 (PXRD) 分析证实了 Cu 和 CuO NPs 的产生。煅烧样品的尖锐和高强度 PXRD 峰证实了形成的 NPs 没有杂质和高结晶度。此外,配体和 Cu(II) 配合物均显示出光致发光特性。此外,我们证明了制备的 1 可以作为单一的固体源前体来形成 Cu 和 CuO NPs。煅烧样品的尖锐和高强度 PXRD 峰证实了形成的 NPs 没有杂质和高结晶度。此外,配体和 Cu(II) 配合物均显示出光致发光特性。此外,我们证明了制备的 1 可以作为单一的固体源前体来形成 Cu 和 CuO NPs。煅烧样品的尖锐和高强度 PXRD 峰证实了形成的 NPs 没有杂质和高结晶度。此外,配体和 Cu(II) 配合物均显示出光致发光特性。此外,我们证明了制备的 1 可以作为单一的固体源前体来形成 Cu 和 CuO NPs。
更新日期:2021-01-01
中文翻译:

烷基取代基对 Cu(II) 复合物中分子构型的影响:使用单一固体源前驱体合成 Cu 和 CuO 纳米颗粒
摘要 一种新的腙型配体4-(吡啶-4基-亚甲基)肼羧酸甲酯(C8H9N3O2; 4-pymc)及其Cu(II)配合物[Cu(4-pymc)2(CH3COO)2(H2O)]( 1) 制备和表征。1的结构包括单体,其中Cu是五配位的,具有扭曲的四方锥体结构。两个单齿乙酸根阴离子的氧原子和来自吡啶环的一对氮原子形成基面。顶端位置被水分子填充。此外,TEM 图像证明使用单源制备的 Cu(II) 复合物通过热分解方法成功制备了 Cu (~35 nm) 和 CuO (~95 nm) 纳米粒子 (NPs),只需改变大气条件(即真空和空气气氛),在此条件下,Cu(II) 配合物在 400 °C 下煅烧 1 小时。此外,TG-DTA 研究表明 1 经历了内分解和放热分解,在空气中形成了作为最终残留物的 CuO。通过粉末 X 射线衍射 (PXRD) 分析证实了 Cu 和 CuO NPs 的产生。煅烧样品的尖锐和高强度 PXRD 峰证实了形成的 NPs 没有杂质和高结晶度。此外,配体和 Cu(II) 配合物均显示出光致发光特性。此外,我们证明了制备的 1 可以作为单一的固体源前体来形成 Cu 和 CuO NPs。煅烧样品的尖锐和高强度 PXRD 峰证实了形成的 NPs 没有杂质和高结晶度。此外,配体和 Cu(II) 配合物均显示出光致发光特性。此外,我们证明了制备的 1 可以作为单一的固体源前体来形成 Cu 和 CuO NPs。煅烧样品的尖锐和高强度 PXRD 峰证实了形成的 NPs 没有杂质和高结晶度。此外,配体和 Cu(II) 配合物均显示出光致发光特性。此外,我们证明了制备的 1 可以作为单一的固体源前体来形成 Cu 和 CuO NPs。



























 京公网安备 11010802027423号
京公网安备 11010802027423号