当前位置:
X-MOL 学术
›
Immunol. Cell Biol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Migratory cues controlling B-lymphocyte trafficking in human lymph nodes.
Immunology and Cell Biology ( IF 4 ) Pub Date : 2020-08-01 , DOI: 10.1111/imcb.12386 Saem Mul Park 1, 2 , Anna Es Brooks 1, 2 , Chun-Jen J Chen 1, 2 , Hilary M Sheppard 1, 2 , Evert Jan Loef 1, 2 , Julie D McIntosh 1, 2 , Catherine E Angel 1, 2 , Claudia J Mansell 1 , Adam Bartlett 3 , Jonathan Cebon 4 , Nigel P Birch 1 , P Rod Dunbar 1, 2
Immunology and Cell Biology ( IF 4 ) Pub Date : 2020-08-01 , DOI: 10.1111/imcb.12386 Saem Mul Park 1, 2 , Anna Es Brooks 1, 2 , Chun-Jen J Chen 1, 2 , Hilary M Sheppard 1, 2 , Evert Jan Loef 1, 2 , Julie D McIntosh 1, 2 , Catherine E Angel 1, 2 , Claudia J Mansell 1 , Adam Bartlett 3 , Jonathan Cebon 4 , Nigel P Birch 1 , P Rod Dunbar 1, 2
Affiliation
|
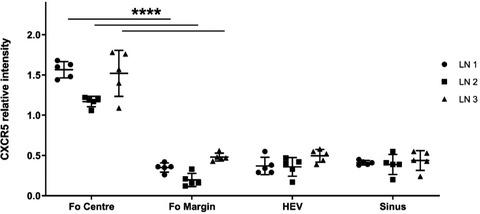
B‐cell migration within lymph nodes (LNs) is crucial to adaptive immune responses. Chemotactic gradients are proposed to drive migration of B cells into follicles, followed by their relocation to specific zones of the follicle during activation, and ultimately egress. However, the molecular drivers of these processes and the cells generating chemotactic signals that affect B cells in human LNs are not well understood. We used immunofluorescence microscopy, flow cytometry and functional assays to study molecular mechanisms of B‐cell migration within human LNs, and found subtle but important differences to previous murine models. In human LNs we find CXCL13 is prominently expressed at the follicular edge, often associated with fibroblastic reticular cells located in these areas, whereas follicular dendritic cells show minimal contribution to CXCL13 expression. Human B cells rapidly downregulate CXCR5 on encountering CXCL13, but recover CXCR5 expression in the CXCL13‐low environment. These data suggest that the CXCL13 gradient in human LNs is likely to be different from that proposed in mice. We also identify CD68+CD11c+PU.1+ tingible body macrophages within both primary and secondary follicles as likely drivers of the sphingosine‐1‐phosphate (S1P) gradient that mediates B‐cell egress from LNs, through their expression of the S1P‐degrading enzyme, S1P lyase. Based on our findings, we present a model of B‐cell migration within human LNs, which has both similarities and interesting differences to that proposed for mice.
中文翻译:

控制人类淋巴结中 B 淋巴细胞运输的迁移线索。
淋巴结 (LN) 内的 B 细胞迁移对适应性免疫反应至关重要。提出趋化梯度来驱动 B 细胞迁移到毛囊中,然后在激活过程中将它们重新定位到毛囊的特定区域,并最终离开。然而,这些过程的分子驱动因素以及产生影响人类 LN 中 B 细胞的趋化信号的细胞尚不清楚。我们使用免疫荧光显微镜、流式细胞术和功能测定来研究人类 LN 中 B 细胞迁移的分子机制,并发现与以前的小鼠模型存在细微但重要的差异。在人类淋巴结中,我们发现 CXCL13 在滤泡边缘显着表达,通常与位于这些区域的成纤维细胞网状细胞相关,而滤泡树突细胞对 CXCL13 表达的贡献很小。人类 B 细胞在遇到 CXCL13 时迅速下调 CXCR5,但在 CXCL13 低环境中恢复 CXCR5 表达。这些数据表明,人类 LN 中的 CXCL13 梯度可能与小鼠中提出的不同。我们还鉴定了 CD68+ CD11c + PU.1 +初级和次级卵泡内的可着色体巨噬细胞可能是 1-磷酸鞘氨醇 (S1P) 梯度的驱动因素,通过 S1P 降解酶 S1P 的表达介导 B 细胞从 LN 流出裂解酶。基于我们的发现,我们提出了一个人类 LN 中 B 细胞迁移的模型,该模型与为小鼠提出的模型既有相似之处,也有有趣的差异。
更新日期:2020-08-01
中文翻译:

控制人类淋巴结中 B 淋巴细胞运输的迁移线索。
淋巴结 (LN) 内的 B 细胞迁移对适应性免疫反应至关重要。提出趋化梯度来驱动 B 细胞迁移到毛囊中,然后在激活过程中将它们重新定位到毛囊的特定区域,并最终离开。然而,这些过程的分子驱动因素以及产生影响人类 LN 中 B 细胞的趋化信号的细胞尚不清楚。我们使用免疫荧光显微镜、流式细胞术和功能测定来研究人类 LN 中 B 细胞迁移的分子机制,并发现与以前的小鼠模型存在细微但重要的差异。在人类淋巴结中,我们发现 CXCL13 在滤泡边缘显着表达,通常与位于这些区域的成纤维细胞网状细胞相关,而滤泡树突细胞对 CXCL13 表达的贡献很小。人类 B 细胞在遇到 CXCL13 时迅速下调 CXCR5,但在 CXCL13 低环境中恢复 CXCR5 表达。这些数据表明,人类 LN 中的 CXCL13 梯度可能与小鼠中提出的不同。我们还鉴定了 CD68+ CD11c + PU.1 +初级和次级卵泡内的可着色体巨噬细胞可能是 1-磷酸鞘氨醇 (S1P) 梯度的驱动因素,通过 S1P 降解酶 S1P 的表达介导 B 细胞从 LN 流出裂解酶。基于我们的发现,我们提出了一个人类 LN 中 B 细胞迁移的模型,该模型与为小鼠提出的模型既有相似之处,也有有趣的差异。



























 京公网安备 11010802027423号
京公网安备 11010802027423号