Catalysis Today ( IF 5.3 ) Pub Date : 2020-08-01 , DOI: 10.1016/j.cattod.2020.07.013 Idia G. Nascimento , William de R. Locatel , Bruno C. Magalhães , Leonardo Travalloni , José L. Zotin , Mônica A.P. da Silva
|
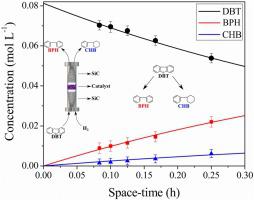
Over the last decades, the regulatory agencies, aiming to limit vehicle emissions, established more stringent fuel specifications, including ultra-low sulfur contents in diesel and gasoline. In such way, hydrodesulfurization (HDS) is a key process in the oil refining scheme for producing low sulfur fuels. The evaluation of the HDS reaction kinetics of refractory compounds, as dibenzothiophene (DBT), is an important tool in the search for improving the activity of usual industrial catalysts. In this context, the main objective of this work was the estimation of kinetic parameters of DBT HDS reactions using CoMoP/Al2O3 and NiMoP/Al2O3 catalysts at operational conditions which provide a wide range of DBT conversions. The estimation results were evaluated using statistical tests as t-Student and chi-square. Good fits to the evaluated experimental data were provided by two power-law models with different levels of detail: one considering the global DBT conversion only and the other considering a reactional scheme with two parallel routes (direct desulfurization to biphenyl and hydrogenation to cyclohexylbenzene). The apparent activation energies found were of the order of 90 to 100 kJ mol−1. Regarding the biphenyl hydrogenation to cyclohexylbenzene, the parameter estimation was hindered because this reaction is expected to be significant at conditions with high DBT conversions only.
中文翻译:

使用 CoMoP/Al2O3 和 NiMoP/Al2O3 的二苯并噻吩加氢脱硫反应动力学
在过去的几十年里,旨在限制车辆排放的监管机构制定了更严格的燃料规范,包括柴油和汽油中的超低硫含量。因此,加氢脱硫 (HDS) 是炼油方案中生产低硫燃料的关键过程。难熔化合物的 HDS 反应动力学评估,如二苯并噻吩 (DBT),是寻找提高常用工业催化剂活性的重要工具。在这种情况下,这项工作的主要目标是使用 CoMoP/Al 2 O 3和 NiMoP/Al 2 O 3估计 DBT HDS 反应的动力学参数催化剂在提供广泛的 DBT 转化率的操作条件下。估计结果使用统计检验作为t -Student 和卡方进行评估。两个具有不同详细程度的幂律模型很好地拟合了评估的实验数据:一个仅考虑全局 DBT 转化,另一个考虑具有两条平行路线的反应方案(直接脱硫为联苯和氢化为环己基苯)。发现的表观活化能大约为 90 到 100 kJ mol -1。关于联苯氢化成环己基苯,参数估计受到阻碍,因为预计该反应仅在 DBT 转化率高的条件下才有意义。



























 京公网安备 11010802027423号
京公网安备 11010802027423号