当前位置:
X-MOL 学术
›
Org. Chem. Front.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Dimerization–cyclization reactions of isocyanoaryl-tethered alkylidenecyclobutanes via a triplet biradical mediated process
Organic Chemistry Frontiers ( IF 5.4 ) Pub Date : 2020-07-31 , DOI: 10.1039/d0qo00878h Le-Yi Tao 1, 2, 3, 4, 5 , Yin Wei 1, 2, 3, 4, 5 , Min Shi 1, 2, 3, 4, 5
Organic Chemistry Frontiers ( IF 5.4 ) Pub Date : 2020-07-31 , DOI: 10.1039/d0qo00878h Le-Yi Tao 1, 2, 3, 4, 5 , Yin Wei 1, 2, 3, 4, 5 , Min Shi 1, 2, 3, 4, 5
Affiliation
|
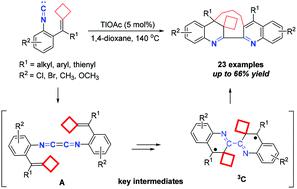
A triplet biradical mediated dimerization–cyclization reaction of isocyanoaryl-tethered alkylidenecyclobutanes has been reported in this paper, giving a new protocol for the construction of macrocyclic skeletons including dihydroquinoline and quinoline units in moderate yields. The reaction proceeded through a key 1,4-diazabutatriene intermediate along with intramolecular redox to produce a biradical intermediate species, which subsequently experienced bond-breaking and -making processes to give the desired product. The reaction mechanism was supported by density functional theory (DFT) calculations.
中文翻译:

异氰基芳基连接的亚烷基环丁烷的二聚化-环化反应通过三重双自由基介导的过程
本文已报道了异氰酸酯基连接的亚烷基环丁烷的三重双自由基介导的二聚化-环化反应,为构建中等收率的包括二氢喹啉和喹啉单元的大环骨架提供了新的协议。该反应通过关键的1,4-二氮杂丁三烯中间体与分子内氧化还原一起进行,以产生双自由基中间体,其随后经历键断裂和制备过程以得到所需产物。反应机理得到密度泛函理论(DFT)计算的支持。
更新日期:2020-09-16
中文翻译:

异氰基芳基连接的亚烷基环丁烷的二聚化-环化反应通过三重双自由基介导的过程
本文已报道了异氰酸酯基连接的亚烷基环丁烷的三重双自由基介导的二聚化-环化反应,为构建中等收率的包括二氢喹啉和喹啉单元的大环骨架提供了新的协议。该反应通过关键的1,4-二氮杂丁三烯中间体与分子内氧化还原一起进行,以产生双自由基中间体,其随后经历键断裂和制备过程以得到所需产物。反应机理得到密度泛函理论(DFT)计算的支持。



























 京公网安备 11010802027423号
京公网安备 11010802027423号