当前位置:
X-MOL 学术
›
ACS Appl. Electron. Mater.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Current–Voltage Characteristics of SiN Membranes in Solution
ACS Applied Electronic Materials ( IF 4.7 ) Pub Date : 2020-07-30 , DOI: 10.1021/acsaelm.0c00479 Itaru Yanagi 1 , Ken-ichi Takeda 1
ACS Applied Electronic Materials ( IF 4.7 ) Pub Date : 2020-07-30 , DOI: 10.1021/acsaelm.0c00479 Itaru Yanagi 1 , Ken-ichi Takeda 1
Affiliation
|
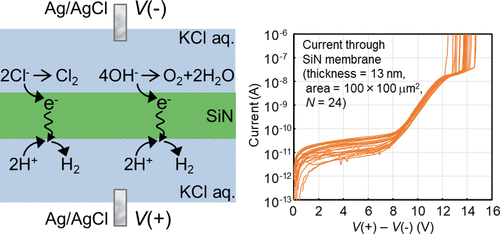
Recently, the dielectric breakdown of insulating membranes in aqueous solution has been utilized to fabricate nanopore sensors for various molecular detection applications. Generally, the breakdown phenomenon of an insulator is thought to be strongly related to the behavior of the current through it. However, until now, the current through an insulating membrane in aqueous solution has rarely been measured. In this study, by using thin SiN membranes with large and precisely defined areas, we achieved accurate and reproducible measurements of the current–voltage (I–V) characteristics of SiN membranes in aqueous solution. From the shape of the I–V curves and their temperature dependence, the carrier conduction process in the membrane was found to be mainly governed by the Poole–Frenkel emission and tunnel conduction of the electrons which are transferred by the ions in aqueous solution to the membrane. In addition, we investigated how the I–V characteristics and breakdown voltage changed in accordance with the changes in the solution pH, solute type, solute concentration, and solvent type. The results were categorized into two cases. In one case, both the I–V characteristics and breakdown voltage changed in accordance with the change in each experimental parameter. In the other case, only the breakdown voltage changed without changes in the I–V characteristics. The most drastic changes in both the I–V characteristics and breakdown voltage were observed when the solvent was changed from water to dimethyl sulfoxide (DMSO), whereas the solute was LiCl. This observation was attributed to the differences in ion species that transfer electrons to and from the membrane. H+, OH–, and Cl– were thought to exchange electrons with the membrane when the solvent was H2O. On the other hand, Li+ and Cl– may have played a role in the electron exchange reaction when the solvent was DMSO.
中文翻译:

溶液中SiN膜的电流-电压特性
近来,绝缘膜在水溶液中的介电击穿已被用于制造用于各种分子检测应用的纳米孔传感器。通常,绝缘子的击穿现象被认为与流过绝缘子的电流的行为密切相关。但是,到目前为止,很少测量通过绝缘膜在水溶液中的电流。在这项研究中,通过使用薄的SiN膜具有大和精确限定的区域,我们实现了电流-电压的精确和可重复的测量(我- V)在水溶液中的SiN膜的特性。从I – V的形状曲线和它们的温度依赖性,发现膜中的载流子传导过程主要受Poole-Frenkel发射和电子的隧穿传导控制,电子通过水溶液中的离子转移到膜上。此外,我们研究了I – V特性和击穿电压如何随溶液pH,溶质类型,溶质浓度和溶剂类型的变化而变化。结果分为两种情况。在一种情况下,无论是我- V特性和击穿电压按照各实验参数的变化而变化。在另一种情况下,仅击穿电压发生变化,而I –V特性。在这两个最剧烈的变化我- V观察特性和击穿电压时,溶剂由水变为二甲亚砜(DMSO),而溶质是氯化锂。该观察结果归因于将电子传递至膜和从膜传递电子的离子种类的差异。ħ +,OH -和Cl -被认为交换的电子与膜当溶剂加入H 2 O.在另一方面,李+和Cl -中的电子交换反应可以发挥了作用,当溶剂为DMSO 。
更新日期:2020-09-22
中文翻译:

溶液中SiN膜的电流-电压特性
近来,绝缘膜在水溶液中的介电击穿已被用于制造用于各种分子检测应用的纳米孔传感器。通常,绝缘子的击穿现象被认为与流过绝缘子的电流的行为密切相关。但是,到目前为止,很少测量通过绝缘膜在水溶液中的电流。在这项研究中,通过使用薄的SiN膜具有大和精确限定的区域,我们实现了电流-电压的精确和可重复的测量(我- V)在水溶液中的SiN膜的特性。从I – V的形状曲线和它们的温度依赖性,发现膜中的载流子传导过程主要受Poole-Frenkel发射和电子的隧穿传导控制,电子通过水溶液中的离子转移到膜上。此外,我们研究了I – V特性和击穿电压如何随溶液pH,溶质类型,溶质浓度和溶剂类型的变化而变化。结果分为两种情况。在一种情况下,无论是我- V特性和击穿电压按照各实验参数的变化而变化。在另一种情况下,仅击穿电压发生变化,而I –V特性。在这两个最剧烈的变化我- V观察特性和击穿电压时,溶剂由水变为二甲亚砜(DMSO),而溶质是氯化锂。该观察结果归因于将电子传递至膜和从膜传递电子的离子种类的差异。ħ +,OH -和Cl -被认为交换的电子与膜当溶剂加入H 2 O.在另一方面,李+和Cl -中的电子交换反应可以发挥了作用,当溶剂为DMSO 。


























 京公网安备 11010802027423号
京公网安备 11010802027423号