Journal of Catalysis ( IF 7.3 ) Pub Date : 2020-07-31 , DOI: 10.1016/j.jcat.2020.07.019 Gengnan Li , Bin Wang , Daniel E. Resasco
|
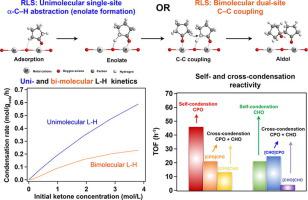
Metal oxides with acid-base properties can be used to catalyze aldol condensation and potentially obtain valuable chemicals from biomass-derived oxygenates. Understanding the reaction mechanisms could enable the development of effective catalysts with controllable selectivity. Here, we investigate the reaction parameters that affect the kinetics and product distribution of self- and cross-aldol condensation of cyclopentanone (CPO) and cyclohexanone (CHO) over CeO2 and ZrO2 catalysts by combining detailed analysis of experiments and DFT calculations. It is well-known that either the deprotonation of an α-C–H that generates an enolate or the C–C coupling that produces the dimers can be rate-limiting step. Indeed, the detailed kinetics analysis shows that the observed data can be fitted by unimolecular or bimolecular Langmuir-Hinshelwood models. Specifically, on the ZrO2 catalyst, the unimolecular α-C–H activation is the rate limiting step for the CPO self-condensation, while the bimolecular C–C coupling limits the CHO self-condensation. By contrast, on CeO2, the bimolecular C–C coupling step seems to be rate-limiting for both CPO and CHO self-condensation reactions. The adsorption parameters derived from kinetics fitting indicate that when the two reactants are co-fed, the surface is preferentially covered by CHO. So, the condensation rate of [CPO]-activated products ([CPO]CPO + [CPO]CHO) decreases in the presence of CHO, due to the competitive adsorption. However, despite a higher surface coverage, CHO is not an effective electrophile because it imposes stronger steric constraints to the formation of the C–C bond than CPO. Therefore, for a given enolate, the product distribution shows that [CPO]CPO > [CPO]CHO and, similarly [CHO]CPO > [CHO]CHO. Likewise, the yields of [CPO]- and [CHO]-activated products in mixed CPO/CHO feeds are similar because the higher surface coverage and stability of the [CHO] enolate is counterbalanced by the inhibition of C–C coupling by steric constraints when CHO is involved. In summary, the overall rank of products observed experimentally can be explained in terms of a combination of the following effects: relative surface coverage of the reactants, steric constraints for the C–C coupling, stability of the enolate, and effectiveness of the electrophile to accept an electron.
中文翻译:

氧化物催化环酮的自缩合和交叉缩合。决定产品分布的动力学相关步骤
具有酸碱性质的金属氧化物可用于催化醛醇缩合,并可能从生物质衍生的含氧化合物中获得有价值的化学物质。了解反应机理可以开发出具有可控选择性的有效催化剂。在这里,我们研究了在CeO 2和ZrO 2上影响环戊酮(CPO)和环己酮(CHO)的自身和交叉醛缩合动力学和产物分布的反应参数。通过将实验的详细分析与DFT计算相结合,来制造催化剂。众所周知,产生烯醇的α-CH的去质子或产生二聚体的CC的耦合都是限速步骤。实际上,详细的动力学分析表明,所观察到的数据可通过单分子或双分子Langmuir-Hinshelwood模型拟合。特别是,在ZrO 2催化剂上,单分子α-C–H活化是CPO自缩合的速率限制步骤,而双分子C–C偶联则限制了CHO自缩合。相比之下,在CeO 2上,双分子C–C偶联步骤似乎是CPO和CHO自缩合反应的速率限制。由动力学拟合得出的吸附参数表明,当两种反应物共同进料时,表面优先被CHO覆盖。因此,由于竞争性吸附,在CHO存在下[CPO]活化产物([CPO] CPO + [CPO] CHO)的缩合速率降低。然而,尽管表面覆盖率更高,但是CHO并不是一种有效的亲电试剂,因为它对C–C键的形成施加了比CPO更强的空间约束。因此,对于给定的烯醇化物,产物分布显示[CPO] CPO> [CPO] CHO,相似地,[CHO] CPO> [CHO] CHO。同样 在CPO / CHO混合饲料中,[CPO]和[CHO]活化产物的产量相似,因为[CHO]烯醇盐的较高表面覆盖率和稳定性可通过CHO受到空间限制而抑制C–C偶联来抵消。参与。总之,可以通过以下作用的组合来解释实验观察到的产物的总体等级:反应物的相对表面覆盖率,CC耦合的空间位阻,烯醇盐的稳定性以及亲电试剂对接受电子。



























 京公网安备 11010802027423号
京公网安备 11010802027423号