International Journal for Parasitology ( IF 4 ) Pub Date : 2020-07-31 , DOI: 10.1016/j.ijpara.2020.06.007 Laura Braden 1 , Dylan Michaud 1 , Okechukwu O Igboeli 1 , Michael Dondrup 2 , Lars Hamre 3 , Sussie Dalvin 4 , Sara L Purcell 1 , Heidi Kongshaug 3 , Christiane Eichner 3 , Frank Nilsen 3 , Mark D Fast 1
|
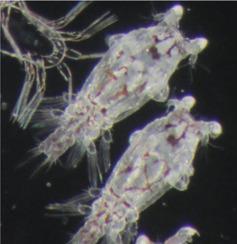
Treatment of infestation by the ectoparasite Lepeophtheirus salmonis relies on a small number of chemotherapeutant treatments that currently meet with limited success. Drugs targeting chitin synthesis have been largely successful against terrestrial parasites where the pathway is well characterised. However, a comparable approach against salmon lice has been, until recently, less successful, likely due to a poor understanding of the chitin synthesis pathway. Post-transcriptional silencing of genes by RNA interference (RNAi) is a powerful method for evaluation of protein function in non-model organisms and has been successfully applied to the salmon louse. In the present study, putative genes coding for enzymes involved in L. salmonis chitin synthesis were characterised after knockdown by RNAi. Nauplii I stage L. salmonis were exposed to double-stranded (ds) RNA specific for several putative non-redundant points in the pathway: glutamine: fructose-6-phosphate aminotransferase (LsGFAT), UDP-N-acetylglucosamine pyrophosphorylase (LsUAP), N-acetylglucosamine phosphate mutase (LsAGM), chitin synthase 1 (LsCHS1), and chitin synthase 2 (LsCHS2). Additionally, we targeted three putative chitin deacetylases (LsCDA4557, 5169 and 5956) by knockdown. Successful knockdown was determined after moulting to the copepodite stage by real-time quantitative PCR (RT-qPCR), while infectivity potential (the number of attached chalimus II compared with the initial number of larvae in the system) was measured after exposure to Atlantic salmon and subsequent development on their host. Compared with controls, infectivity potential was not compromised in dsAGM, dsCHS2, dsCDA4557, or dsCDA5169 groups. In contrast, there was a significant effect in the dsUAP-treated group. However, of most interest was the treatment with dsGFAT, dsCHS1, dsCHS1+2, and dsCDA5956, which resulted in complete abrogation of infectivity, despite apparent compensatory mechanisms in the chitin synthesis pathway as detected by qPCR. There appeared to be a common phenotypic effect in these groups, characterised by significant aberrations in appendage morphology and an inability to swim. Ultrastructurally, dsGFAT showed a significantly distorted procuticle without distinct exo/endocuticle and intermittent electron dense (i.e. chitin) inclusions, and together with dsUAP and dsCHS1, indicated delayed entry to the pre-moult phase.
中文翻译:

通过 RNA 干扰介导的传染性消除所揭示的鲑鱼虱几丁质合成途径中关键酶的鉴定。
外寄生虫鲑鱼鳞翅目感染的治疗依赖于少数目前取得有限成功的化疗药物治疗。靶向几丁质合成的药物在对抗陆生寄生虫方面取得了很大的成功,其中该途径已得到充分表征。然而,直到最近,针对鲑鱼虱的类似方法一直不太成功,这可能是由于对几丁质合成途径的了解不足。通过 RNA 干扰 (RNAi) 进行基因转录后沉默是一种评估非模式生物蛋白质功能的有效方法,并已成功应用于鲑鱼虱子。在本研究中,推测的编码与L.salmonis 相关的酶的基因几丁质合成在被 RNAi 敲低后表征。无节幼体 I 阶段的鲑鱼暴露于双链 (ds) RNA,该 RNA 对途径中的几个假定的非冗余点具有特异性:谷氨酰胺:6-磷酸果糖转氨酶( LsGFAT )、UDP-N-乙酰氨基葡萄糖焦磷酸化酶( LsUAP )、N-乙酰氨基葡萄糖磷酸变位酶( LsAGM )、几丁质合酶 1 ( LsCHS1 ) 和几丁质合酶 2 ( LsCHS2 )。此外,我们针对三种假定的几丁质脱乙酰酶(LsCDA4557、5169和5956) 击倒。通过实时定量 PCR (RT-qPCR) 在蜕皮到桡足阶段后确定成功击倒,同时在暴露于大西洋鲑鱼后测量感染潜力(附着的 chalimus II 数量与系统中的初始幼虫数量相比)并在其主机上进行后续开发。与对照相比,dsAGM、dsCHS2、dsCDA4557 或 dsCDA5169 组的感染潜力没有受到影响。相比之下,dsUAP 治疗组有显着效果。然而,最令人感兴趣的是用 dsGFAT、dsCHS1、dsCHS1+2 和 dsCDA5956 处理,尽管 qPCR 检测到几丁质合成途径中有明显的补偿机制,但它导致感染性完全消除。在这些组中似乎有一个共同的表型效应,其特征是附肢形态显着畸变和不能游泳。超微结构上,dsGFAT 显示出明显扭曲的前表皮,没有明显的外/内表皮和间歇性电子致密(即几丁质)包裹体,与 dsUAP 和 dsCHS1 一起表明进入换羽前阶段的延迟。


























 京公网安备 11010802027423号
京公网安备 11010802027423号