Biochemical and Biophysical Research Communications ( IF 3.1 ) Pub Date : 2020-07-31 , DOI: 10.1016/j.bbrc.2020.06.091 Mei Zheng 1 , Xiaohan Zhang 1 , Xiao Min 1 , Ningning Sun 1 , Kyeong-Man Kim 1
|
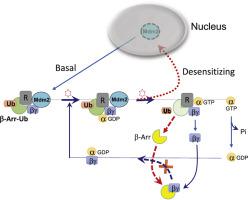
Desensitization of G protein-coupled receptors (GPCRs) represents a gradual attenuation of receptor responsiveness by continuous or repeated exposure to agonists. The most widely accepted molecular mechanism responsible for desensitization is that of GRK2-mediated receptor phosphorylation followed by association with β-arrestins. However, in most cases, this mechanism cannot explain the desensitization of GPCRs. In this study, we investigated whether there exists a direct correlation between desensitization and certain cellular events that commonly observed with desensitizing receptors. Our study showed that constitutive ubiquitination of β-arrestin, accompanied by nuclear to cytoplasmic translocation of Mdm2, was observed in cells expressing desensitizing GPCRs (dopamine D3 receptor, K149C-dopamine D2 receptor, β2 adrenoceptor, and lysophosphatidic acid receptor 1). In contrast, Mdm2 was observed in the nucleus in cells expressing non-desensitizing GPCRs (dopamine D2 receptor, C147K-dopamine D3 receptor, and dopamine D4 receptor). Molecular manipulation to convert the characteristics of the dopamine D4 receptor from non-desensitizing to desensitizing changed the status of subcellular localization of Mdm2 from nuclear to cytoplasmic. With repeated agonist treatments of desensitizing receptors, Mdm2 translocated from cytoplasm to nucleus, resulting in the deubiquitination of β-arrestins. This study suggests that the property of a receptor that causes a change in subcellular localization of Mdm2, from the nuclear to cytoplasmic, could be used as a biomarker to predict the desensitization of a receptor.
中文翻译:

Mdm2的细胞质募集是经历脱敏的G蛋白偶联受体的共同特征。
G蛋白偶联受体(GPCR)的脱敏性表示通过连续或反复暴露于激动剂使受体反应性逐渐减弱。引起脱敏的最广泛接受的分子机制是GRK2介导的受体磷酸化,然后与β-arrestin缔合。但是,在大多数情况下,该机制无法解释GPCR的脱敏性。在这项研究中,我们研究了脱敏与脱敏受体通常观察到的某些细胞事件之间是否存在直接相关性。我们的研究表明,在表达脱敏GPCR(多巴胺D 3受体,K149C-多巴胺D的细胞)中观察到β-arrestin的组成型泛素化,伴随着Mdm2的核向细胞质移位。2受体,β 2肾上腺素受体,和溶血磷脂酸受体1)。相反,在表达非脱敏GPCR(多巴胺D 2受体,C147K-多巴胺D 3受体和多巴胺D 4受体)的细胞核中观察到Mdm2 。分子操纵以转化多巴胺D 4的特性从非脱敏到脱敏的受体改变了Mdm2的亚细胞定位状态,从核到细胞质。通过对脱敏受体的反复激动剂治疗,Mdm2从细胞质转移到细胞核,导致β-arrestin的去泛素化。这项研究表明,引起Mdm2亚细胞定位从核到胞质变化的受体特性可以用作预测受体脱敏的生物标记。



























 京公网安备 11010802027423号
京公网安备 11010802027423号