Letters in Drug Design & Discovery ( IF 1 ) Pub Date : 2020-07-31 , DOI: 10.2174/1570180817666191224094049 Shahrzad Ghafary 1 , Hamid Nadri 2 , Mohammad Mahdavi 3 , Alireza Moradi 2 , Tahmineh Akbarzadeh 1 , Mohammad Sharifzadeh 4 , Najmeh Edraki 5 , Farshad Homayouni Moghadam 6 , Mohsen Amini 1
|
Background: Acetylcholine deficiency in the hippocampus and cortex, aggregation of amyloid-beta, and beta-secretase overactivity have been introduced as the main reasons in the formation of Alzheimer’s disease.
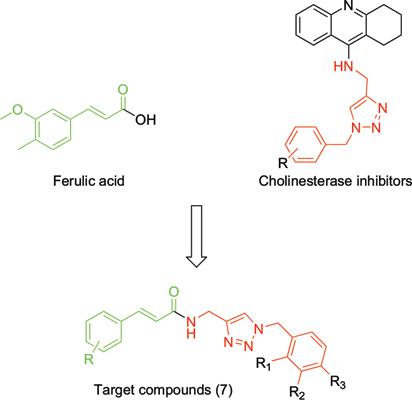
Objective: A new series of cinnamic derived acids linked to 1-benzyl-1,2,3-triazole moiety were designed, synthesized, and evaluated for their acetylcholinesterase (AChE) and butyrylcholinesterase (BuChE) inhibitory activities.
Methods: Colorimetric Ellman’s method was used for the determination of IC50% of AchE and BuChE inhibitory activity. The kinetic studies, neuroprotective activity, BACE1 inhibitory activity, evaluation of inhibitory potency on Aβ1-42 self-aggregation induced by AchE, and docking study were performed for studying the mechanism of action.
Results: Some of the synthesized compounds, compound 7b-4 ((E)-3-(3,4-dimethoxyphenyl)-N-((1- (4-fluorobenzyl)-1H-1,2,3-triazole-4-yl) methyl) acrylamide) depicted the most potent acetylcholinesterase inhibitory activities ( IC50 = 5.27 μM ) and compound 7a-1 (N- ( (1- benzyl- 1H- 1, 2, 3- triazole - 4-yl) methyl) cinnamamide) demonstrated the most potent butyrylcholinesterase inhibitory activities (IC50 = 1.75 μM). Compound 7b-4 showed neuroprotective and β-secretase (BACE1) inhibitory activitiy. In vivo studies of compound 7b-4 in Scopolamine-induced dysfunction confirmed memory improvement.
Conclusion: It should be noted that molecular modeling (compounds 7b-4 and 7a-1) and kinetic studies (compounds 7a-1 and 7b-4) showed that these synthesis compounds interacted simultaneously with both the catalytic site (CS) and peripheral anionic site (PAS) of AChE and BuChE.
中文翻译:

肉桂酸衍生物的抗胆碱酯酶活性:体外,体内生物学评估和对接研究。
背景:海马和皮质的乙酰胆碱缺乏症,淀粉样β蛋白的聚集和β-分泌酶过度活跃已被引入,这是阿尔茨海默氏病形成的主要原因。
目的:设计,合成和链接到一系列新的肉桂酸衍生的1-苄基-1,2,3-三唑部分的乙酰胆碱酯酶(AChE)和丁酰胆碱酯酶(BuChE)抑制活性。
方法:采用比色Ellman法测定AchE的IC50%和BuChE的抑制活性。进行了动力学研究,神经保护活性,BACE1抑制活性,对AchE诱导的Aβ1-42自聚集的抑制能力评价以及对接研究以研究其作用机理。
结果:一些合成的化合物,化合物7b-4((E)-3-(3,4-二甲氧基苯基)-N-((1-(4-氟苄基)-1H-1,2,3-三唑-4 -yl)甲基)丙烯酰胺)表现出最强的乙酰胆碱酯酶抑制活性(IC50 = 5.27μM)和化合物7a-1(N-((1-苄基-1H-1、2、3-三唑-4-基)甲基)肉桂酰胺)表现出最强的丁酰胆碱酯酶抑制活性(IC50 = 1.75μM)。化合物7b-4显示出神经保护和β-分泌酶(BACE1)抑制活性。Scopolamine诱导的功能障碍的化合物7b-4的体内研究证实了记忆力的改善。
结论:应注意的是,分子建模(化合物7b-4和7a-1)和动力学研究(化合物7a-1和7b-4)表明,这些合成化合物同时与催化位点(CS)和外围阴离子相互作用AChE和BuChE的网站(PAS)。


























 京公网安备 11010802027423号
京公网安备 11010802027423号