Letters in Drug Design & Discovery ( IF 1 ) Pub Date : 2020-07-31 , DOI: 10.2174/1570180817666200203125010 Hoda Sharifi 1 , Ahmad Ebadi 2 , Meysam Soleimani 1
|
Background: Kinesins and tubulin inhibitors have attracted researchers’ attention as hopeful targets for achieving effective anticancer agents. Dihydropyrimidine-2-ones (DHPMs) inhibit motor proteins Eg5 in the polymerization process of tubulin, also scaffold bearing benzothiazole heterocycle can block tubulin polymerization/depolymerization.
Objective: In this study, the cytotoxic effects and molecular modeling of newly synthesized derivatives of DHPM that were designed by the Scaffold-hopping approach were investigated as potential dual-inhibitors of Eg5 and tubulin.
Methods: We investigated the cytotoxic effects of DHPMs derivatives by MTT assay and measureing the Caspase 3 activity. Also, molecular modeling studies were performed by AutoDock4 and GROMACS 4.5.6.
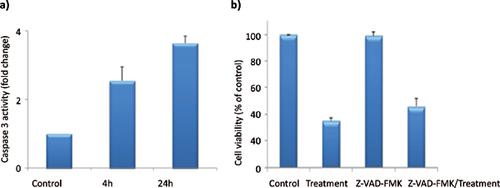
Results: According to the results, the d2 derivative (IC50 = 68.58 ± 7, SI = 2.57) eliminates MDA-MB- 231 cells in a dose-dependent manner through caspase-dependent and caspase-independent cell death pathways. Molecular docking studies revealed that the d2 compound could interact with both Eg5 and tubulin key residues. MD simulation also demonstrated the stability of the studied ligand-receptor complexes during the 30 ns of the production run. The effectiveness of substitutions at C4 of the DHPM ring was obtained 4-acetoxy-phenyl, 4-methoxyphenyl, and 4-nitrophenyl, respectively.
Conclusion: The findings of the present study provide evidence that DHPM C5 amide derivatives bearing benzothiazole ring might be considered as promising lead compounds for the discovery of novel and multi-target antitumor agents.
中文翻译:

3,4-二氢嘧啶-2(1H)-one衍生物作为细胞毒性药物对乳腺癌的生物学评价和分子模型
背景:驱动蛋白和微管蛋白抑制剂作为获得有效抗癌药的希望靶点吸引了研究人员的注意力。二氢嘧啶-2-酮(DHPM)在微管蛋白的聚合过程中抑制运动蛋白Eg5,同时带有苯并噻唑杂环的支架也可阻止微管蛋白的聚合/解聚。
目的:在本研究中,研究了通过支架跳跃法设计的DHPM新合成衍生物作为Eg5和微管蛋白的潜在双重抑制剂的细胞毒性作用和分子模型。
方法:我们通过MTT分析和测量Caspase 3活性研究了DHPMs衍生物的细胞毒性作用。另外,分子建模研究是通过AutoDock4和GROMACS 4.5.6进行的。
结果:根据结果,d2衍生物(IC50 = 68.58±7,SI = 2.57)通过caspase依赖性和caspase依赖性细胞死亡途径以剂量依赖性方式消除MDA-MB-231细胞。分子对接研究表明,d2化合物可与Eg5和微管蛋白关键残基相互作用。MD模拟还证明了在生产过程的30 ns内研究的配体-受体复合物的稳定性。分别获得4-乙酰氧基-苯基,4-甲氧基苯基和4-硝基苯基在DHPM环的C4处取代的有效性。
结论:本研究的发现提供了证据,即带有苯并噻唑环的DHPM C5酰胺衍生物可能被认为是发现新型和多靶点抗肿瘤药物的有前途的先导化合物。



























 京公网安备 11010802027423号
京公网安备 11010802027423号