当前位置:
X-MOL 学术
›
Bull. Korean Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
DABCO‐Catalyzed the Synthesis of Densely Functionalized Cyclohexanones in a Benign Manner
Bulletin of the Korean Chemical Society ( IF 1.7 ) Pub Date : 2020-07-30 , DOI: 10.1002/bkcs.12067 Nourallah Hazeri 1 , Mojtaba Lashkari 2 , Maryam Fatahpour 1 , Mahla Sheikhveisi 1
Bulletin of the Korean Chemical Society ( IF 1.7 ) Pub Date : 2020-07-30 , DOI: 10.1002/bkcs.12067 Nourallah Hazeri 1 , Mojtaba Lashkari 2 , Maryam Fatahpour 1 , Mahla Sheikhveisi 1
Affiliation
|
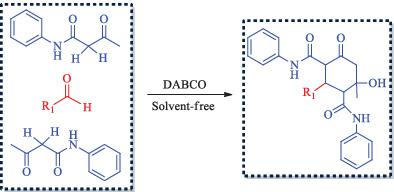
In this study, a robust DABCO‐catalyzed method has been reported for the reaction of readily available material, including various aromatic/aliphatic aldehydes and acetoacetanilide affording N,N′‐diaryl‐2‐aryl‐6‐hydroxy‐6‐methyl‐4‐oxocyclohexane‐1,3‐dicarboxamides. The reaction proceeds at 50 °C under solvent‐free conditions via a one‐pot pseudo‐three‐component reaction to yield functionalized cyclohexanones containing four quaternary stereogenic centers. Simple reaction conditions, moderate‐to‐high yields of product, no need of column chromatographic purification, eco‐friendliness, shorter reaction time, and simple work‐up has transformed this method an interesting route to the available procedures. The stereoselectivity of compounds was envisaged with crystallography and NMR spectroscopy.
中文翻译:

DABCO以良性方式催化了密集功能化环己酮的合成
在这项研究中,已经报道了一种可靠的DABCO催化方法,可用于容易获得的材料(包括各种芳族/脂肪醛和乙酰乙酰胺)的反应,从而提供N,N'-二芳基-2-芳基-6-羟基-6-甲基-4 -氧代环己烷-1,3-二羧酸酰胺。在50℃下无溶剂的条件下通过一釜反应进行伪三组分反应生成包含四个季生立体中心的官能化环己酮。简单的反应条件,中等至高收率的产品,无需柱色谱纯化,生态友好,更短的反应时间以及简单的后处理,使该方法成为了一种可利用的有趣方法。可以通过晶体学和NMR光谱研究化合物的立体选择性。
更新日期:2020-07-30
中文翻译:

DABCO以良性方式催化了密集功能化环己酮的合成
在这项研究中,已经报道了一种可靠的DABCO催化方法,可用于容易获得的材料(包括各种芳族/脂肪醛和乙酰乙酰胺)的反应,从而提供N,N'-二芳基-2-芳基-6-羟基-6-甲基-4 -氧代环己烷-1,3-二羧酸酰胺。在50℃下无溶剂的条件下通过一釜反应进行伪三组分反应生成包含四个季生立体中心的官能化环己酮。简单的反应条件,中等至高收率的产品,无需柱色谱纯化,生态友好,更短的反应时间以及简单的后处理,使该方法成为了一种可利用的有趣方法。可以通过晶体学和NMR光谱研究化合物的立体选择性。


























 京公网安备 11010802027423号
京公网安备 11010802027423号