当前位置:
X-MOL 学术
›
J. Mol. Struct.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
An empirical and theoretical description of Schistosoma japonicum glutathione transferase inhibition by bromosulfophthalein and indanyloxyacetic acid 94
Journal of Molecular Structure ( IF 3.8 ) Pub Date : 2021-01-01 , DOI: 10.1016/j.molstruc.2020.128892 Kagiso Pooe , Roland Worth , Emmanuel Amarachi Iwuchukwu , Heini W. Dirr , Ikechukwu Achilonu
Journal of Molecular Structure ( IF 3.8 ) Pub Date : 2021-01-01 , DOI: 10.1016/j.molstruc.2020.128892 Kagiso Pooe , Roland Worth , Emmanuel Amarachi Iwuchukwu , Heini W. Dirr , Ikechukwu Achilonu
|
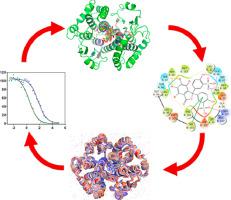
Abstract The inhibition of the 26-kDa glutathione transferase from Schistosoma japonicum (Sj26GST) by bromosulfophthalein (BSP) and indanaloxyacetic acid-94 (IAA-94) was investigated using a systematic combination of empirical and theoretical approaches. Activity studies showed a decrease in the specific activity of Sj26GST from 13.14 µmol/min/mg to 0.13 µmol/min/mg and 2.07 µmol/min/mg in the presence of BSP and IAA-94, respectively. IC50 results suggest that BSP is a more potent inhibitor (IC50 = 2.83 µM) relative to IAA-94 (IC50 = 61.95 µM). Extrinsic 1-anilinonaphthalene-8-sulfonic acid (ANS) fluorescence showed a decrease in the fluorescent quantum yield in the presence of BSP and IAA-94, which indicates a displacement of ANS from buried hydrophobic clefts in Sj26GST, one notable sight being the L-site (dimer interface). The thermodynamics underlying the IAA-94:Sj26GST interaction suggested that the interaction was energetically-favourable, entropically-driven and occurred with a stoichiometry of one IAA-94 molecule per Sj26GST dimer. By using praziquantel (PZQ) for comparison., Induced-fit ligand docking studies indicate that all three ligands prefer binding at the dimer interface. A 50 ns molecular dynamic simulation further showed that these ligands were stabilized by H-bonds, water-bridges, van der Waals and electrostatic interactions with side chains and water molecules within 4 A of the ligands. Free binding energy calculations showed that the binding energies were comparable and are energetically favourable. Overall, the binding of the BSP and IAA-94 resulted in decreased compactness and decrease in conformational dynamics of the protein, suggesting that the inhibition of Sj26GST by BSP and IAA-94 may be non-competitive due to allosteric effect.
中文翻译:

溴磺酞和茚满氧乙酸抑制日本血吸虫谷胱甘肽转移酶的经验和理论描述 94
摘要 使用经验和理论方法的系统组合研究了溴磺酞 (BSP) 和茚满氧乙酸-94 (IAA-94) 对来自日本血吸虫 (Sj26GST) 的 26-kDa 谷胱甘肽转移酶的抑制作用。活性研究表明,在 BSP 和 IAA-94 存在下,Sj26GST 的比活性分别从 13.14 µmol/min/mg 降低到 0.13 µmol/min/mg 和 2.07 µmol/min/mg。IC50 结果表明,相对于 IAA-94 (IC50 = 61.95 µM),BSP 是一种更有效的抑制剂 (IC50 = 2.83 µM)。在 BSP 和 IAA-94 存在下,外源性 1-anilinonaphthalene-8-磺酸 (ANS) 荧光显示荧光量子产率降低,这表明 ANS 从 Sj26GST 中埋藏的疏水裂缝中置换出来,一个值得注意的现象是 L -site(二聚体界面)。IAA-94:Sj26GST 相互作用背后的热力学表明,这种相互作用是能量有利的、熵驱动的,并且以每个 Sj26GST 二聚体一个 IAA-94 分子的化学计量发生。通过使用吡喹酮 (PZQ) 进行比较,诱导拟合配体对接研究表明,所有三种配体都更喜欢在二聚体界面结合。50 ns 分子动力学模拟进一步表明,这些配体通过 H 键、水桥、范德华力和与配体 4 A 内的侧链和水分子的静电相互作用而稳定。自由结合能计算表明结合能具有可比性并且在能量上是有利的。总体而言,BSP 和 IAA-94 的结合导致蛋白质的致密性降低和构象动力学降低,
更新日期:2021-01-01
中文翻译:

溴磺酞和茚满氧乙酸抑制日本血吸虫谷胱甘肽转移酶的经验和理论描述 94
摘要 使用经验和理论方法的系统组合研究了溴磺酞 (BSP) 和茚满氧乙酸-94 (IAA-94) 对来自日本血吸虫 (Sj26GST) 的 26-kDa 谷胱甘肽转移酶的抑制作用。活性研究表明,在 BSP 和 IAA-94 存在下,Sj26GST 的比活性分别从 13.14 µmol/min/mg 降低到 0.13 µmol/min/mg 和 2.07 µmol/min/mg。IC50 结果表明,相对于 IAA-94 (IC50 = 61.95 µM),BSP 是一种更有效的抑制剂 (IC50 = 2.83 µM)。在 BSP 和 IAA-94 存在下,外源性 1-anilinonaphthalene-8-磺酸 (ANS) 荧光显示荧光量子产率降低,这表明 ANS 从 Sj26GST 中埋藏的疏水裂缝中置换出来,一个值得注意的现象是 L -site(二聚体界面)。IAA-94:Sj26GST 相互作用背后的热力学表明,这种相互作用是能量有利的、熵驱动的,并且以每个 Sj26GST 二聚体一个 IAA-94 分子的化学计量发生。通过使用吡喹酮 (PZQ) 进行比较,诱导拟合配体对接研究表明,所有三种配体都更喜欢在二聚体界面结合。50 ns 分子动力学模拟进一步表明,这些配体通过 H 键、水桥、范德华力和与配体 4 A 内的侧链和水分子的静电相互作用而稳定。自由结合能计算表明结合能具有可比性并且在能量上是有利的。总体而言,BSP 和 IAA-94 的结合导致蛋白质的致密性降低和构象动力学降低,



























 京公网安备 11010802027423号
京公网安备 11010802027423号