Chem ( IF 23.5 ) Pub Date : 2020-07-30 , DOI: 10.1016/j.chempr.2020.06.036 Xiang Chen , Xin Shen , Ting-Zheng Hou , Rui Zhang , Hong-Jie Peng , Qiang Zhang
|
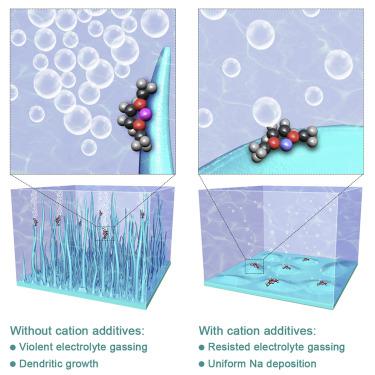
Building stable electrolytes is one of the key technologies for sodium (Na)-metal batteries as the reactive nature and the dendritic growth of Na-metal anodes. Herein, a paradigmatic and rational strategy of cation additive was proposed to stabilize electrolytes for Na metal batteries. Three principles, including the electrode potential of introduced cations, the lowest unoccupied molecular orbital energy level decrease of solvents after coordinating with cations, and the interaction strength between cations and solvents, were proved through first-principles calculations and molecular dynamics simulations. Li+ was predicted to be a good cation additive candidate for Na metal batteries. Finite element method simulations, in situ optical microscopic observations, and electrochemical tests further validated the resisted Na dendritic growth due to the electrostatic shield effect and enhanced electrolyte stability after introducing Li+ additives. The proven cation additive strategy affords emerging chances for rational electrolyte design for stable and safe Na metal batteries.
中文翻译:

离子溶剂化学启发的阳离子添加剂策略,用于稳定钠金属电池的电解质
建立稳定的电解质是钠(Na)金属电池的关键技术之一,因为钠金属阳极的反应性和树枝状生长。本文提出了一种范式合理的阳离子添加剂策略来稳定钠金属电池的电解质。通过第一性原理计算和分子动力学模拟证明了三个原理,包括引入的阳离子的电极电位,与阳离子配位后溶剂的最低未占据分子轨道能级降低,以及阳离子与溶剂之间的相互作用强度。预计Li +是Na金属电池的良好阳离子添加剂。有限元方法模拟,原位光学显微镜观察和电化学测试进一步验证了由于引入Li +添加剂后的静电屏蔽效应和增强的电解质稳定性,可抵抗Na树突生长。成熟的阳离子添加剂策略为合理设计电解质以提供稳定而安全的Na金属电池提供了新的机会。



























 京公网安备 11010802027423号
京公网安备 11010802027423号