当前位置:
X-MOL 学术
›
Org. Process Res. Dev.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Improved scale-up synthesis and purification of clinical asthma candidate MIDD0301.
Organic Process Research & Development ( IF 3.4 ) Pub Date : 2020-07-29 , DOI: 10.1021/acs.oprd.0c00200 Daniel E Knutson 1 , Rashid Roni 1 , Yeunus Mian 1 , James M Cook 1 , Douglas C Stafford 1, 2 , Leggy A Arnold 1, 2
Organic Process Research & Development ( IF 3.4 ) Pub Date : 2020-07-29 , DOI: 10.1021/acs.oprd.0c00200 Daniel E Knutson 1 , Rashid Roni 1 , Yeunus Mian 1 , James M Cook 1 , Douglas C Stafford 1, 2 , Leggy A Arnold 1, 2
Affiliation
|
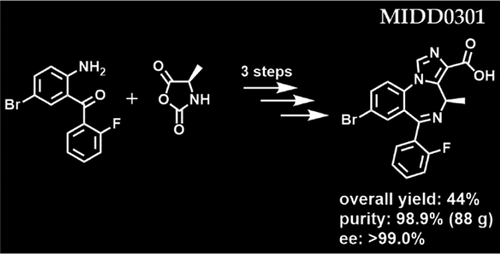
We report an improved and scalable synthesis of MIDD0301, a positive GABAA receptor modulator that is under development as oral and inhaled treatments for asthma. In contrast to other benzodiazepines in clinical use, MIDD0301 is a chiral compound that has limited brain absorption. The starting material to generate MIDD0301 is 2-amino-5-bromo-2′-fluorobenzophenone, which has a nonbasic nitrogen due to electron-withdrawing substituents in the ortho and para positions, reducing its reactivity toward activated carboxylic acids. Investigations of peptide coupling reagents on a multigram scale resulted in moderate yields due to incomplete conversions. Second, the basic conditions used for the formation of the seven-membered 1,4-diazepine ring resulted in racemization of the chiral center. We found that neutral conditions comparable to the pKa of the primary amine were sufficient to support the formation of the intramolecular imine but did not enable the simultaneous removal of the protecting group. Both difficulties were overcome with the application of the N-carboxyanhydride of d-alanine. Activated in the presence of an acid, this compound reacted with nonbasic 2-amino-5-bromo-2′-fluorobenzophenone and formed the 1,4-diazepine upon neutralization with triethylamine. Carefully designed workup procedures and divergent solubility of the synthesic intermediates in solvents and solvent combinations were utilized to eliminate the need for column chromatography. To improve compatibility with large-scale reactors, temperature-controlled slow addition of reagents generated the imidazodiazepine at −20 °C. All intermediates were isolated with a purity of >97% and impurities were identified and quantified. After the final hydrolysis step, MIDD0301 was isolated in a 44% overall yield and a purity of 98.9% after recrystallization. The enantiomeric excess was greater than 99.0%.
中文翻译:

改进的临床哮喘候选药物 MIDD0301 的放大合成和纯化。
我们报告了 MIDD0301 的改进和可扩展合成,这是一种正 GABA A受体调节剂,正在开发用于哮喘的口服和吸入治疗。与临床使用的其他苯二氮卓类药物相比,MIDD0301 是一种脑吸收有限的手性化合物。生成 MIDD0301 的起始材料是 2-amino-5-bromo-2'-fluoro二苯甲酮,由于邻位和对位的吸电子取代基,它具有非碱性氮位置,降低其对活化羧酸的反应性。由于转化不完全,对多克规模的肽偶联试剂的研究导致中等产量。其次,用于形成七元 1,4-二氮杂环的基本条件导致手性中心的外消旋化。我们发现与伯胺的 p K a相当的中性条件足以支持分子内亚胺的形成,但不能同时去除保护基团。通过应用d的N-羧酸酐克服了这两个困难-丙氨酸。在酸存在下活化,该化合物与非碱性 2-氨基-5-溴-2'-氟二苯甲酮反应并在与三乙胺中和后形成 1,4-二氮杂。精心设计的后处理程序和合成中间体在溶剂和溶剂组合中的不同溶解度被用来消除对柱色谱的需要。为了提高与大型反应器的兼容性,在-20°C 下,通过温度控制缓慢添加试剂生成咪唑二氮杂。所有中间体均以 >97% 的纯度分离,杂质得到鉴定和定量。在最后的水解步骤之后,MIDD0301以44%的总收率和重结晶后的98.9%的纯度被分离。对映体过量大于99.0%。
更新日期:2020-08-21
中文翻译:

改进的临床哮喘候选药物 MIDD0301 的放大合成和纯化。
我们报告了 MIDD0301 的改进和可扩展合成,这是一种正 GABA A受体调节剂,正在开发用于哮喘的口服和吸入治疗。与临床使用的其他苯二氮卓类药物相比,MIDD0301 是一种脑吸收有限的手性化合物。生成 MIDD0301 的起始材料是 2-amino-5-bromo-2'-fluoro二苯甲酮,由于邻位和对位的吸电子取代基,它具有非碱性氮位置,降低其对活化羧酸的反应性。由于转化不完全,对多克规模的肽偶联试剂的研究导致中等产量。其次,用于形成七元 1,4-二氮杂环的基本条件导致手性中心的外消旋化。我们发现与伯胺的 p K a相当的中性条件足以支持分子内亚胺的形成,但不能同时去除保护基团。通过应用d的N-羧酸酐克服了这两个困难-丙氨酸。在酸存在下活化,该化合物与非碱性 2-氨基-5-溴-2'-氟二苯甲酮反应并在与三乙胺中和后形成 1,4-二氮杂。精心设计的后处理程序和合成中间体在溶剂和溶剂组合中的不同溶解度被用来消除对柱色谱的需要。为了提高与大型反应器的兼容性,在-20°C 下,通过温度控制缓慢添加试剂生成咪唑二氮杂。所有中间体均以 >97% 的纯度分离,杂质得到鉴定和定量。在最后的水解步骤之后,MIDD0301以44%的总收率和重结晶后的98.9%的纯度被分离。对映体过量大于99.0%。



























 京公网安备 11010802027423号
京公网安备 11010802027423号