Insect Biochemistry and Molecular Biology ( IF 3.8 ) Pub Date : 2020-07-29 , DOI: 10.1016/j.ibmb.2020.103438 Jacob J Weber 1 , Michael R Kanost 1 , Maureen J Gorman 1
|
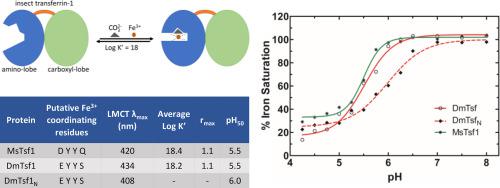
Transferrins belong to an ancient family of extracellular proteins. The best-characterized transferrins are mammalian proteins that function in iron sequestration or iron transport; they accomplish these functions by having a high-affinity iron-binding site in each of their two homologous lobes. Insect hemolymph transferrins (Tsf1s) also function in iron sequestration and transport; however, sequence-based predictions of their iron-binding residues have suggested that most Tsf1s have a single, lower-affinity iron-binding site. To reconcile the apparent contradiction between the known physiological functions and predicted biochemical properties of Tsf1s, we purified and characterized the iron-binding properties of Drosophila melanogaster Tsf1 (DmTsf1), Manduca sexta Tsf1 (MsTsf1), and the amino-lobe of DmTsf1 (DmTsf1N). Using UV–Vis spectroscopy, we found that these proteins bind iron, but they exhibit shifts in their spectra compared to mammalian transferrins. Through equilibrium dialysis experiments, we determined that DmTsf1 and MsTsf1 bind only one ferric ion; their affinity for iron is high (log K’ = 18), but less than that of the well-characterized mammalian transferrins (log K’ ~ 20); and they release iron under moderately acidic conditions (pH50 = 5.5). Iron release analysis of DmTsf1N suggested that iron binding in the amino-lobe is stabilized by the carboxyl-lobe. These findings will be critical for elucidating the mechanisms of Tsf1 function in iron sequestration and transport in insects.
中文翻译:

来自黑腹果蝇和 Manduca sexta 的转铁蛋白-1 的铁结合和释放特性:对昆虫铁稳态的影响。
转铁蛋白属于一个古老的细胞外蛋白家族。最具特征的转铁蛋白是在铁螯合或铁转运中起作用的哺乳动物蛋白质。它们通过在其两个同源叶中的每一个中具有高亲和力的铁结合位点来实现这些功能。昆虫血淋巴转铁蛋白 (Tsf1s) 在铁螯合和运输中也起作用;然而,对其铁结合残基的基于序列的预测表明,大多数 Tsf1s 具有单一的、亲和力较低的铁结合位点。为了调和 Tsf1s 已知生理功能和预测生化特性之间的明显矛盾,我们纯化并表征了黑腹果蝇Tsf1 (DmTsf1)、Manduca sexta的铁结合特性Tsf1 (MsTsf1) 和 DmTsf1 的氨基叶 (DmTsf1 N )。使用紫外-可见光谱,我们发现这些蛋白质与铁结合,但与哺乳动物转铁蛋白相比,它们的光谱发生了变化。通过平衡透析实验,我们确定 DmTsf1 和 MsTsf1 仅结合一个三价铁离子;它们对铁的亲和力很高(log K ' = 18),但低于已充分表征的哺乳动物转铁蛋白(log K ' ~ 20);它们在中等酸性条件下(pH 50 = 5.5)释放铁。DmTsf1 N 的铁释放分析表明氨基叶中的铁结合由羧基叶稳定。这些发现对于阐明 Tsf1 在昆虫铁螯合和转运中的功能机制至关重要。



























 京公网安备 11010802027423号
京公网安备 11010802027423号