Current Organic Synthesis ( IF 1.8 ) Pub Date : 2020-07-31 , DOI: 10.2174/1570179417666200410170237 Aparna Wadhwa 1 , Faraat Ali 1 , Sana Parveen 2 , Robin Kumar 1 , Gyanendra N Singh 1
|
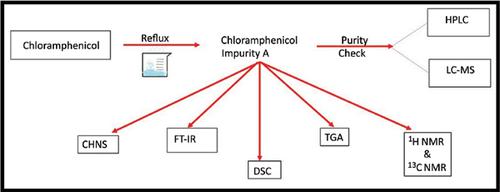
Objective: The main aim of the present work is to synthesize chloramphenicol impurity A (CLRMIMP- A) in the purest form and its subsequent characterization by using a panel of sophisticated analytical techniques (LC-MS, DSC, TGA, NMR, FTIR, HPLC, and CHNS) to provide as a reference standard mentioned in most of the international compendiums, including IP, BP, USP, and EP. The present synthetic procedure has not been disclosed anywhere in the prior art.
Methods: A simple, cheaper, and new synthesis method was described for the preparation of CLRM-IMP-A. It was synthesized and characterized by FTIR, DSC, TGA, NMR (1H and 13C), LC-MS, CHNS, and HPLC.
Results: CLRM-IMP-A present in drugs and dosage form can alter the therapeutic effects and adverse reaction of a drug considerably, it is mandatory to have a precise method for the estimation of impurities to safeguard the public health. Under these circumstances, the presence of CLRM-IMP-A in chloramphenicol (CLRM) requires strict quality control to satisfy the specified regulatory limit. The synthetic impurity obtained was in the pure form to provide a certified reference standard or working standard to stakeholders with defined potency.
Conclusion: The present research describes a novel technique for the synthesis of pharmacopoeial impurity, which can help in checking/controlling the quality of the CLRM in the international markets.
中文翻译:

氯霉素杂质A的合成,分析表征和光谱研究,用于氯霉素的质量控制及其根据国际简编的规定。
目的:本研究的主要目的是使用一组复杂的分析技术(LC-MS,DSC,TGA,NMR,FTIR,HPLC)以最纯净的形式合成氯霉素杂质A(CLRMIMP- A)及其后续表征(和CHNS)作为参考标准,以在大多数国际摘要中提及,包括IP,BP,USP和EP。在现有技术中的任何地方都没有公开本合成方法。
方法:描述了一种简单,便宜,新的合成方法来制备CLRM-IMP-A。通过FTIR,DSC,TGA,NMR(1H和13C),LC-MS,CHNS和HPLC对其进行了合成和表征。
结果:存在于药物和剂型中的CLRM-IMP-A可以大大改变药物的治疗效果和不良反应,因此必须使用精确的杂质估计方法来保护公众健康。在这种情况下,氯霉素(CLRM)中CLRM-IMP-A的存在需要严格的质量控制,以满足指定的法规限制。所获得的合成杂质为纯净形式,可向利益相关者提供经认证的参考标准或工作标准,并具有确定的效力。
结论:本研究描述了一种合成药典杂质的新技术,该技术可帮助检查/控制国际市场上CLRM的质量。

























 京公网安备 11010802027423号
京公网安备 11010802027423号