当前位置:
X-MOL 学术
›
Biomacromolecules
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Tunable Fast Relaxation in Imine-Based Nanofibrillar Hydrogels Stimulates Cell Response through TRPV4 Activation.
Biomacromolecules ( IF 6.2 ) Pub Date : 2020-07-27 , DOI: 10.1021/acs.biomac.0c00850 Amin Liu 1 , Kai Wu 1 , Suping Chen 1 , Chengheng Wu 1 , Dong Gao 1 , Lu Chen 1 , Dan Wei 1 , Hongrong Luo 1 , Jing Sun 1 , Hongsong Fan 1
Biomacromolecules ( IF 6.2 ) Pub Date : 2020-07-27 , DOI: 10.1021/acs.biomac.0c00850 Amin Liu 1 , Kai Wu 1 , Suping Chen 1 , Chengheng Wu 1 , Dong Gao 1 , Lu Chen 1 , Dan Wei 1 , Hongrong Luo 1 , Jing Sun 1 , Hongsong Fan 1
Affiliation
|
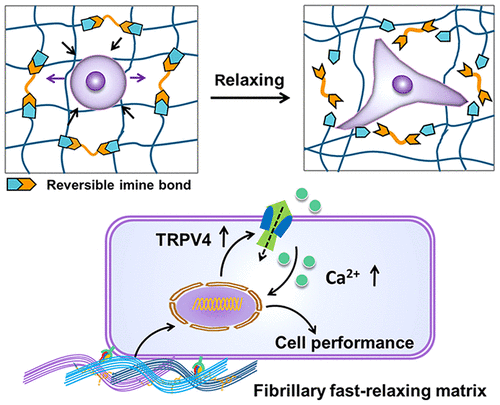
As a key mechanical signal of natural extracellular matrix (ECM), stress relaxation plays an essential role in cell fate decision. However, the biomimetic matrix with fast stress relaxation and its cellular response mechanism have received little attention. Meanwhile, the nanofibrillar architecture which is conductive to mechanical transduction has invariably been ignored in the previous viscoelastic matrix design. Herein, by introducing a dynamic covalent imine bond into a physically cross-linked collagen hydrogel, we prepared bionic fast-relaxing nanofibrillar hydrogels with relaxation time less than 10 s. Through a single control of imine bond content, we realized fine-tuning of the relaxation rate while maintaining a constant initial modulus and fiber density. Using MC3T3-E1 cells as a model, we then proved that the nanofibrillar matrix with fast relaxation mechanics can effectively promote cell spreading and differentiation. In particular, TRPV4 as a molecular sensor of matrix viscoelasticity was demonstrated to regulate cell fate on the nanofibrillar hydrogels by mediating calcium influx. It is expected that the material design principle combining both nanofibrillar structure and tunable fast-relaxation can provide a more broadly adaptable materials platform for simulating natural ECM mechanical cues, and the investigation of the TRPV4 ion channel mediated cellular response will facilitate discovery of more fundamental mechanisms in tissue growth and development.
中文翻译:

在基于亚胺的纳米原纤维水凝胶中可调节的快速松弛通过TRPV4激活刺激细胞反应。
作为天然细胞外基质(ECM)的关键机械信号,应力松弛在细胞命运决定中起着至关重要的作用。然而,具有快速应力松弛的仿生基质及其细胞应答机制很少受到关注。同时,在先前的粘弹性基体设计中,总是会忽略有助于机械转导的纳米原纤维结构。本文中,通过将动态共价亚胺键引入物理交联的胶原水凝胶中,我们制备了松弛时间小于10 s的仿生快速松弛纳米原纤维水凝胶。通过亚胺键含量的单一控制,我们实现了松弛率的微调,同时保持了恒定的初始模量和纤维密度。以MC3T3-E1电池为模型,然后我们证明了具有快速松弛机制的纳米原纤维基质可以有效地促进细胞扩散和分化。特别是,TRPV4作为基质粘弹性的分子传感器已被证明可通过介导钙内流来调节纳米原纤维水凝胶上的细胞命运。预期结合纳米纤丝结构和可调快速松弛的材料设计原理可以为模拟自然ECM机械线索提供更广泛适用的材料平台,并且TRPV4离子通道介导的细胞反应的研究将有助于发现更多的基本机理在组织生长和发育中。已经证明,TRPV4作为基质粘弹性的分子传感器可通过介导钙内流来调节纳米原纤维水凝胶上的细胞命运。预期结合纳米纤丝结构和可调快速松弛的材料设计原理可以为模拟自然ECM机械线索提供更广泛适用的材料平台,并且TRPV4离子通道介导的细胞反应的研究将有助于发现更多的基本机理在组织生长和发育中。已经证明,TRPV4作为基质粘弹性的分子传感器可通过介导钙内流来调节纳米原纤维水凝胶上的细胞命运。预期结合纳米纤丝结构和可调快速松弛的材料设计原理可以为模拟自然ECM机械线索提供更广泛适用的材料平台,并且TRPV4离子通道介导的细胞反应的研究将有助于发现更多的基本机理在组织生长和发育中。
更新日期:2020-09-14
中文翻译:

在基于亚胺的纳米原纤维水凝胶中可调节的快速松弛通过TRPV4激活刺激细胞反应。
作为天然细胞外基质(ECM)的关键机械信号,应力松弛在细胞命运决定中起着至关重要的作用。然而,具有快速应力松弛的仿生基质及其细胞应答机制很少受到关注。同时,在先前的粘弹性基体设计中,总是会忽略有助于机械转导的纳米原纤维结构。本文中,通过将动态共价亚胺键引入物理交联的胶原水凝胶中,我们制备了松弛时间小于10 s的仿生快速松弛纳米原纤维水凝胶。通过亚胺键含量的单一控制,我们实现了松弛率的微调,同时保持了恒定的初始模量和纤维密度。以MC3T3-E1电池为模型,然后我们证明了具有快速松弛机制的纳米原纤维基质可以有效地促进细胞扩散和分化。特别是,TRPV4作为基质粘弹性的分子传感器已被证明可通过介导钙内流来调节纳米原纤维水凝胶上的细胞命运。预期结合纳米纤丝结构和可调快速松弛的材料设计原理可以为模拟自然ECM机械线索提供更广泛适用的材料平台,并且TRPV4离子通道介导的细胞反应的研究将有助于发现更多的基本机理在组织生长和发育中。已经证明,TRPV4作为基质粘弹性的分子传感器可通过介导钙内流来调节纳米原纤维水凝胶上的细胞命运。预期结合纳米纤丝结构和可调快速松弛的材料设计原理可以为模拟自然ECM机械线索提供更广泛适用的材料平台,并且TRPV4离子通道介导的细胞反应的研究将有助于发现更多的基本机理在组织生长和发育中。已经证明,TRPV4作为基质粘弹性的分子传感器可通过介导钙内流来调节纳米原纤维水凝胶上的细胞命运。预期结合纳米纤丝结构和可调快速松弛的材料设计原理可以为模拟自然ECM机械线索提供更广泛适用的材料平台,并且TRPV4离子通道介导的细胞反应的研究将有助于发现更多的基本机理在组织生长和发育中。



























 京公网安备 11010802027423号
京公网安备 11010802027423号