当前位置:
X-MOL 学术
›
J. Heterocycl. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
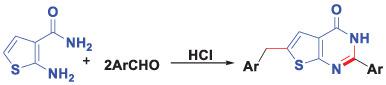
An efficient synthesis of 6‐benzyl‐2‐arylthieno[2,3‐d]pyrimidin‐4(3H)‐ones catalyzed by HCl involving a Friedel‐Crafts alkylation reaction
Journal of Heterocyclic Chemistry ( IF 2.4 ) Pub Date : 2020-07-27 , DOI: 10.1002/jhet.4107 Wan‐Chen Pan 1 , Yi‐Chun Wang 1 , Tuan‐Jie Li 1 , Jian‐Quan Liu 1 , Xiang‐Shan Wang 1
Journal of Heterocyclic Chemistry ( IF 2.4 ) Pub Date : 2020-07-27 , DOI: 10.1002/jhet.4107 Wan‐Chen Pan 1 , Yi‐Chun Wang 1 , Tuan‐Jie Li 1 , Jian‐Quan Liu 1 , Xiang‐Shan Wang 1
Affiliation
|
Aldehyde could undergo not only the subsequent condensation and cyclization with 2‐aminothiophene‐3‐carboxamide to build a pyrimidine ring, but also a Friedel‐Crafts alkylation reaction with thiophene moiety to give unexpected 6‐benzyl‐2‐arylthieno[2,3‐d]pyrimidin‐4(3H)‐ ones in good yields catalyzed by concentrated HCl.
中文翻译:

HCl催化的Friedel-Crafts烷基化反应有效合成6-苄基-2-芳基噻吩并[2,3-d]嘧啶-4(3H)-酮
乙醛不仅可以随后与2-氨基噻吩-3-羧酰胺进行缩合和环化以构建一个嘧啶环,而且还可以与噻吩部分进行Friedel-Crafts烷基化反应,从而产生意想不到的6-苄基-2-芳基噻吩并[2,3- d ]嘧啶-4(3 H)-浓HCl催化收率高。
更新日期:2020-07-27
中文翻译:

HCl催化的Friedel-Crafts烷基化反应有效合成6-苄基-2-芳基噻吩并[2,3-d]嘧啶-4(3H)-酮
乙醛不仅可以随后与2-氨基噻吩-3-羧酰胺进行缩合和环化以构建一个嘧啶环,而且还可以与噻吩部分进行Friedel-Crafts烷基化反应,从而产生意想不到的6-苄基-2-芳基噻吩并[2,3- d ]嘧啶-4(3 H)-浓HCl催化收率高。



























 京公网安备 11010802027423号
京公网安备 11010802027423号