当前位置:
X-MOL 学术
›
Asian J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
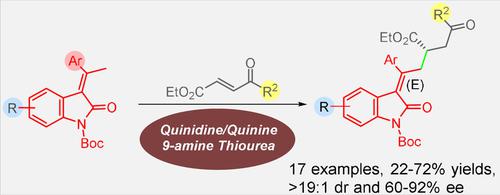
Bifunctional Cinchona Alkaloid Catalyzed Vinylogous Michael Reaction of 3–Alkylideneoxindole with 4‐Oxo‐enoates: A Route to Chiral γ‐Keto Alkylideneoxindole Esters
Asian Journal of Organic Chemistry ( IF 2.7 ) Pub Date : 2020-07-28 , DOI: 10.1002/ajoc.202000227 Rajesh Kumar 1 , Manish K. Jaiswal 1 , Ravi P. Singh 1
Asian Journal of Organic Chemistry ( IF 2.7 ) Pub Date : 2020-07-28 , DOI: 10.1002/ajoc.202000227 Rajesh Kumar 1 , Manish K. Jaiswal 1 , Ravi P. Singh 1
Affiliation
|
A bifunctional cinchona alkaloid‐catalyzed vinylogous Michael addition reaction involving 3‐alkylideneoxindoles as the vinylogous nucleophiles and various β‐aroyl acrylates as the Michael acceptor is described. The reaction provides expedient access to functionalised chiral γ‐keto alkylideneoxindole esters in good to high yields and stereoselectivities (up to >19 : 1 d.r. and 92% ee).
中文翻译:

双功能金鸡纳生物碱催化3-烷基亚氧吲哚与4-氧-烯酸酯的乙烯基迈克尔反应:通向手性γ-酮基烷基亚氧吲哚酯的途径
描述了一种双功能金鸡纳生物碱催化的乙烯基迈克尔加成反应,其中涉及3-亚烷基亚氧吲哚作为乙烯基亲核试剂,以及各种β-芳酰基丙烯酸酯作为迈克尔受体。该反应可方便地获得官能化的手性γ-酮亚烷基氧亚吲哚酯,产率高,立体选择性好(高达> 19:1 dr和92%ee)。
更新日期:2020-07-28
中文翻译:

双功能金鸡纳生物碱催化3-烷基亚氧吲哚与4-氧-烯酸酯的乙烯基迈克尔反应:通向手性γ-酮基烷基亚氧吲哚酯的途径
描述了一种双功能金鸡纳生物碱催化的乙烯基迈克尔加成反应,其中涉及3-亚烷基亚氧吲哚作为乙烯基亲核试剂,以及各种β-芳酰基丙烯酸酯作为迈克尔受体。该反应可方便地获得官能化的手性γ-酮亚烷基氧亚吲哚酯,产率高,立体选择性好(高达> 19:1 dr和92%ee)。


























 京公网安备 11010802027423号
京公网安备 11010802027423号