Structure ( IF 5.7 ) Pub Date : 2020-07-28 , DOI: 10.1016/j.str.2020.07.003 Jiuyang Liu 1 , Zhaoyu Xue 2 , Yi Zhang 1 , Kendra R Vann 1 , Xiaobing Shi 2 , Tatiana G Kutateladze 1
|
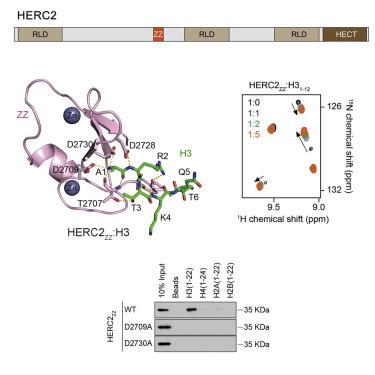
Human ubiquitin ligase HERC2, a component of the DNA repair machinery, has been linked to neurological diseases and cancer. Here, we show that the ZZ domain of HERC2 (HERC2ZZ) binds to histone H3 tail and tolerates posttranslational modifications commonly present in H3. The crystal structure of the HERC2ZZ:H3 complex provides the molecular basis for this interaction and highlights a critical role of the negatively charged site of HERC2ZZ in capturing of A1 of H3. NMR, mutagenesis, and fluorescence data reveal that HERC2ZZ binds to H3 and the N-terminal tail of SUMO1, a previously reported ligand of HERC2ZZ, with comparable affinities. Like H3, the N-terminal tail of SUMO1 occupies the same negatively charged site of HERC2ZZ in the crystal structure of the complex, although in contrast to H3 it adopts an α-helical conformation. Our data suggest that HERC2ZZ may play a role in mediating the association of HERC2 with chromatin.
中文翻译:

HERC2 的 ZZ 结构域与组蛋白 H3 和 SUMO1 结合的结构洞察。
人类泛素连接酶 HERC2 是 DNA 修复机制的一个组成部分,与神经系统疾病和癌症有关。在这里,我们展示了 HERC2 的 ZZ 结构域 (HERC2 ZZ ) 与组蛋白 H3 尾部结合并耐受 H3 中常见的翻译后修饰。HERC2 ZZ :H3 复合物的晶体结构为这种相互作用提供了分子基础,并突出了 HERC2 ZZ带负电荷位点在捕获 H3 的 A1 中的关键作用。核磁共振、诱变和荧光数据显示 HERC2 ZZ与 H3 和SUMO1的 N 末端尾部结合,SUMO1 是先前报道的 HERC2 ZZ配体,具有可比的亲和力。与 H3 一样,SUMO1 的 N 末端尾部在复合物的晶体结构中占据与 HERC2 ZZ相同的带负电荷的位点,尽管与 H3 相比,它采用α-螺旋构象。我们的数据表明 HERC2 ZZ可能在介导 HERC2 与染色质的关联中发挥作用。



























 京公网安备 11010802027423号
京公网安备 11010802027423号