当前位置:
X-MOL 学术
›
ACS Chem. Biol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Robust Sequence Determinants of α-Synuclein Toxicity in Yeast Implicate Membrane Binding.
ACS Chemical Biology ( IF 4 ) Pub Date : 2020-07-27 , DOI: 10.1021/acschembio.0c00339 Robert W Newberry 1 , Taylor Arhar 2 , Jean Costello 3 , George C Hartoularos 3 , Alison M Maxwell 2 , Zun Zar Chi Naing 3 , Maureen Pittman 3 , Nishith R Reddy 3 , Daniel M C Schwarz 2 , Douglas R Wassarman 2 , Taia S Wu 2 , Daniel Barrero 3 , Christa Caggiano 3 , Adam Catching 3 , Taylor B Cavazos 3 , Laurel S Estes 3 , Bryan Faust 3 , Elissa A Fink 3 , Miriam A Goldman 3 , Yessica K Gomez 3 , M Grace Gordon 3 , Laura M Gunsalus 3 , Nick Hoppe 3 , Maru Jaime-Garza 3 , Matthew C Johnson 3 , Matthew G Jones 3 , Andrew F Kung 3 , Kyle E Lopez 3 , Jared Lumpe 3 , Calla Martyn 3 , Elizabeth E McCarthy 3 , Lakshmi E Miller-Vedam 3 , Erik J Navarro 3 , Aji Palar 3 , Jenna Pellegrino 3 , Wren Saylor 3 , Christina A Stephens 3 , Jack Strickland 3 , Hayarpi Torosyan 3 , Stephanie A Wankowicz 3 , Daniel R Wong 3 , Garrett Wong 3 , Sy Redding 4 , Eric D Chow 4 , William F DeGrado 1 , Martin Kampmann 4, 5, 6
ACS Chemical Biology ( IF 4 ) Pub Date : 2020-07-27 , DOI: 10.1021/acschembio.0c00339 Robert W Newberry 1 , Taylor Arhar 2 , Jean Costello 3 , George C Hartoularos 3 , Alison M Maxwell 2 , Zun Zar Chi Naing 3 , Maureen Pittman 3 , Nishith R Reddy 3 , Daniel M C Schwarz 2 , Douglas R Wassarman 2 , Taia S Wu 2 , Daniel Barrero 3 , Christa Caggiano 3 , Adam Catching 3 , Taylor B Cavazos 3 , Laurel S Estes 3 , Bryan Faust 3 , Elissa A Fink 3 , Miriam A Goldman 3 , Yessica K Gomez 3 , M Grace Gordon 3 , Laura M Gunsalus 3 , Nick Hoppe 3 , Maru Jaime-Garza 3 , Matthew C Johnson 3 , Matthew G Jones 3 , Andrew F Kung 3 , Kyle E Lopez 3 , Jared Lumpe 3 , Calla Martyn 3 , Elizabeth E McCarthy 3 , Lakshmi E Miller-Vedam 3 , Erik J Navarro 3 , Aji Palar 3 , Jenna Pellegrino 3 , Wren Saylor 3 , Christina A Stephens 3 , Jack Strickland 3 , Hayarpi Torosyan 3 , Stephanie A Wankowicz 3 , Daniel R Wong 3 , Garrett Wong 3 , Sy Redding 4 , Eric D Chow 4 , William F DeGrado 1 , Martin Kampmann 4, 5, 6
Affiliation
|
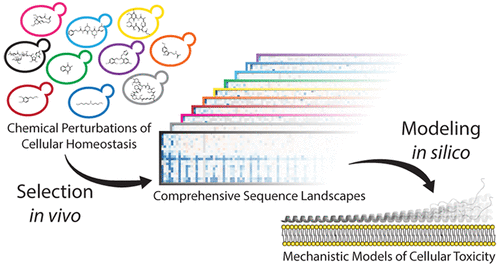
Protein conformations are shaped by cellular environments, but how environmental changes alter the conformational landscapes of specific proteins in vivo remains largely uncharacterized, in part due to the challenge of probing protein structures in living cells. Here, we use deep mutational scanning to investigate how a toxic conformation of α-synuclein, a dynamic protein linked to Parkinson’s disease, responds to perturbations of cellular proteostasis. In the context of a course for graduate students in the UCSF Integrative Program in Quantitative Biology, we screened a comprehensive library of α-synuclein missense mutants in yeast cells treated with a variety of small molecules that perturb cellular processes linked to α-synuclein biology and pathobiology. We found that the conformation of α-synuclein previously shown to drive yeast toxicity—an extended, membrane-bound helix—is largely unaffected by these chemical perturbations, underscoring the importance of this conformational state as a driver of cellular toxicity. On the other hand, the chemical perturbations have a significant effect on the ability of mutations to suppress α-synuclein toxicity. Moreover, we find that sequence determinants of α-synuclein toxicity are well described by a simple structural model of the membrane-bound helix. This model predicts that α-synuclein penetrates the membrane to constant depth across its length but that membrane affinity decreases toward the C terminus, which is consistent with orthogonal biophysical measurements. Finally, we discuss how parallelized chemical genetics experiments can provide a robust framework for inquiry-based graduate coursework.
中文翻译:

酵母涉及膜结合中α-突触核蛋白毒性的稳健序列决定因素。
蛋白质构象由细胞环境塑造,但环境变化如何改变体内特定蛋白质的构象景观仍然很大程度上没有特征,部分原因是探测活细胞中的蛋白质结构的挑战。在这里,我们使用深度突变扫描来研究 α-突触核蛋白(一种与帕金森病相关的动态蛋白质)的毒性构象如何响应细胞蛋白质稳态的扰动。在加州大学旧金山分校定量生物学综合计划研究生课程的背景下,我们筛选了酵母细胞中 α-突触核蛋白错义突变体的综合库,这些突变体用各种小分子处理,这些小分子干扰了与 α-突触核蛋白生物学相关的细胞过程和病理生物学。我们发现,先前显示出驱动酵母毒性的 α-突触核蛋白的构象——一种扩展的膜结合螺旋——在很大程度上不受这些化学扰动的影响,强调这种构象状态作为细胞毒性驱动因素的重要性。另一方面,化学扰动对突变抑制 α-突触核蛋白毒性的能力有显着影响。此外,我们发现 α-突触核蛋白毒性的序列决定因素可以通过膜结合螺旋的简单结构模型得到很好的描述。该模型预测 α-突触核蛋白在其长度上穿透膜至恒定深度,但膜亲和力向 C 末端降低,这与正交生物物理测量一致。最后,我们讨论了并行化学遗传学实验如何为基于探究的研究生课程提供强大的框架。化学扰动对突变抑制 α-突触核蛋白毒性的能力有显着影响。此外,我们发现通过膜结合螺旋的简单结构模型很好地描述了 α-突触核蛋白毒性的序列决定因素。该模型预测 α-突触核蛋白在其长度上穿透膜至恒定深度,但膜亲和力向 C 末端降低,这与正交生物物理测量一致。最后,我们讨论了并行化学遗传学实验如何为基于探究的研究生课程提供强大的框架。化学扰动对突变抑制 α-突触核蛋白毒性的能力有显着影响。此外,我们发现 α-突触核蛋白毒性的序列决定因素可以通过膜结合螺旋的简单结构模型得到很好的描述。该模型预测 α-突触核蛋白在其长度上穿透膜至恒定深度,但膜亲和力向 C 末端降低,这与正交生物物理测量一致。最后,我们讨论了并行化学遗传学实验如何为基于探究的研究生课程提供强大的框架。该模型预测 α-突触核蛋白在其长度上穿透膜至恒定深度,但膜亲和力向 C 末端降低,这与正交生物物理测量一致。最后,我们讨论了并行化学遗传学实验如何为基于探究的研究生课程提供强大的框架。该模型预测 α-突触核蛋白在其长度上穿透膜至恒定深度,但膜亲和力向 C 末端降低,这与正交生物物理测量一致。最后,我们讨论了并行化学遗传学实验如何为基于探究的研究生课程提供强大的框架。
更新日期:2020-08-21
中文翻译:

酵母涉及膜结合中α-突触核蛋白毒性的稳健序列决定因素。
蛋白质构象由细胞环境塑造,但环境变化如何改变体内特定蛋白质的构象景观仍然很大程度上没有特征,部分原因是探测活细胞中的蛋白质结构的挑战。在这里,我们使用深度突变扫描来研究 α-突触核蛋白(一种与帕金森病相关的动态蛋白质)的毒性构象如何响应细胞蛋白质稳态的扰动。在加州大学旧金山分校定量生物学综合计划研究生课程的背景下,我们筛选了酵母细胞中 α-突触核蛋白错义突变体的综合库,这些突变体用各种小分子处理,这些小分子干扰了与 α-突触核蛋白生物学相关的细胞过程和病理生物学。我们发现,先前显示出驱动酵母毒性的 α-突触核蛋白的构象——一种扩展的膜结合螺旋——在很大程度上不受这些化学扰动的影响,强调这种构象状态作为细胞毒性驱动因素的重要性。另一方面,化学扰动对突变抑制 α-突触核蛋白毒性的能力有显着影响。此外,我们发现 α-突触核蛋白毒性的序列决定因素可以通过膜结合螺旋的简单结构模型得到很好的描述。该模型预测 α-突触核蛋白在其长度上穿透膜至恒定深度,但膜亲和力向 C 末端降低,这与正交生物物理测量一致。最后,我们讨论了并行化学遗传学实验如何为基于探究的研究生课程提供强大的框架。化学扰动对突变抑制 α-突触核蛋白毒性的能力有显着影响。此外,我们发现通过膜结合螺旋的简单结构模型很好地描述了 α-突触核蛋白毒性的序列决定因素。该模型预测 α-突触核蛋白在其长度上穿透膜至恒定深度,但膜亲和力向 C 末端降低,这与正交生物物理测量一致。最后,我们讨论了并行化学遗传学实验如何为基于探究的研究生课程提供强大的框架。化学扰动对突变抑制 α-突触核蛋白毒性的能力有显着影响。此外,我们发现 α-突触核蛋白毒性的序列决定因素可以通过膜结合螺旋的简单结构模型得到很好的描述。该模型预测 α-突触核蛋白在其长度上穿透膜至恒定深度,但膜亲和力向 C 末端降低,这与正交生物物理测量一致。最后,我们讨论了并行化学遗传学实验如何为基于探究的研究生课程提供强大的框架。该模型预测 α-突触核蛋白在其长度上穿透膜至恒定深度,但膜亲和力向 C 末端降低,这与正交生物物理测量一致。最后,我们讨论了并行化学遗传学实验如何为基于探究的研究生课程提供强大的框架。该模型预测 α-突触核蛋白在其长度上穿透膜至恒定深度,但膜亲和力向 C 末端降低,这与正交生物物理测量一致。最后,我们讨论了并行化学遗传学实验如何为基于探究的研究生课程提供强大的框架。

























 京公网安备 11010802027423号
京公网安备 11010802027423号