当前位置:
X-MOL 学术
›
ACS Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Enantioselective 1,2-Addition of α-Aminoalkyl Radical to Aldehydes via Visible-Light Photoredox Initiated Chiral Oxazaborolidinium Ion Catalysis
ACS Catalysis ( IF 12.9 ) Pub Date : 2020-07-27 , DOI: 10.1021/acscatal.0c02443 Jae Yeon Kim 1 , Yea Suel Lee 1 , Yuna Choi 1 , Do Hyun Ryu 1
ACS Catalysis ( IF 12.9 ) Pub Date : 2020-07-27 , DOI: 10.1021/acscatal.0c02443 Jae Yeon Kim 1 , Yea Suel Lee 1 , Yuna Choi 1 , Do Hyun Ryu 1
Affiliation
|
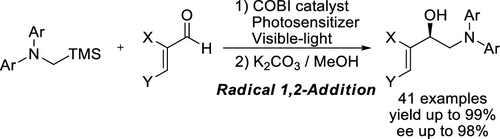
Enantioselective 1,2-addition reaction of α-aminoalkyl radical to α,β-unsaturated or aromatic aldehydes to synthesize highly optically active β-amino alcohols has been developed. In the presence of chiral oxazaborolidinium ion catalyst and photosensitizer, the reaction provides desired β-amino allylic or benzylic alcohols with high yields (up to 99%) and high enantioselectivities (up to 98% ee).
中文翻译:

通过可见光氧化还原引发的手性恶唑硼烷鎓离子催化对醛的对映选择性1,2-加成的α-氨基烷基自由基
已经开发了α-氨基烷基与α,β-不饱和或芳族醛的对映选择性1,2-加成反应,以合成高度旋光活性的β-氨基醇。在手性恶唑硼烷鎓离子催化剂和光敏剂的存在下,反应以高产率(高达99%)和高对映选择性(高达98%ee)提供所需的β-氨基烯丙基或苄基醇。
更新日期:2020-09-20
中文翻译:

通过可见光氧化还原引发的手性恶唑硼烷鎓离子催化对醛的对映选择性1,2-加成的α-氨基烷基自由基
已经开发了α-氨基烷基与α,β-不饱和或芳族醛的对映选择性1,2-加成反应,以合成高度旋光活性的β-氨基醇。在手性恶唑硼烷鎓离子催化剂和光敏剂的存在下,反应以高产率(高达99%)和高对映选择性(高达98%ee)提供所需的β-氨基烯丙基或苄基醇。



























 京公网安备 11010802027423号
京公网安备 11010802027423号