当前位置:
X-MOL 学术
›
J. Neurosci. Res.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Cytoskeletal regulation of synaptogenesis in a model of human fetal brain development.
Journal of Neuroscience Research ( IF 4.2 ) Pub Date : 2020-07-26 , DOI: 10.1002/jnr.24692 Emily Wilson 1 , Taylor Rudisill 1 , Brenna Kirk 1 , Colin Johnson 1 , Paige Kemper 1 , Karen Newell-Litwa 1
Journal of Neuroscience Research ( IF 4.2 ) Pub Date : 2020-07-26 , DOI: 10.1002/jnr.24692 Emily Wilson 1 , Taylor Rudisill 1 , Brenna Kirk 1 , Colin Johnson 1 , Paige Kemper 1 , Karen Newell-Litwa 1
Affiliation
|
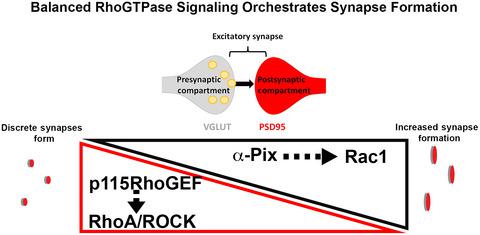
Excitatory synapse formation begins in mid‐fetal gestation. However, due to our inability to image fetal synaptogenesis, the initial formation of synapses remains understudied. The recent development of human fetal brain spheroids provides access to this critical period of synapse formation. Using human neurons and brain spheroids, we address how altered actin regulation impacts the formation of excitatory synapses during fetal brain development. Prior to synapse formation, inhibition of RhoA kinase (ROCK) signaling promotes neurite elongation and branching. In addition to increasing neural complexity, ROCK inhibition increases the length of protrusions along the neurite, ultimately promoting excitatory synapse formation in human cortical brain spheroids. A corresponding increase in Rac1‐driven actin polymerization drives this increase in excitatory synaptogenesis. Using STORM super‐resolution microscopy, we demonstrate that actomyosin regulators, including the Rac1 regulator, α‐PIX, and the RhoA regulator, p115‐RhoGEF, localize to nascent excitatory synapses, where they preferentially localize to postsynaptic compartments. These results demonstrate that coordinated RhoGTPase activities underlie the initial formation of excitatory synapses and identify critical cytoskeletal regulators of early synaptogenic events.
中文翻译:

人类胎儿大脑发育模型中突触发生的细胞骨架调节。
兴奋性突触形成始于中期胎儿妊娠。然而,由于我们无法对胎儿突触发生进行成像,突触的初始形成仍未得到充分研究。人类胎儿脑球体的最新发展为突触形成的这一关键时期提供了途径。使用人类神经元和大脑球体,我们解决了肌动蛋白调节的改变如何影响胎儿大脑发育过程中兴奋性突触的形成。在突触形成之前,抑制 RhoA 激酶 (ROCK) 信号会促进神经突伸长和分支。除了增加神经复杂性之外,ROCK 抑制还会增加沿神经突的突起长度,最终促进人皮质脑球体中兴奋性突触的形成。Rac1 驱动的肌动蛋白聚合的相应增加推动了兴奋性突触发生的增加。使用 STORM 超分辨率显微镜,我们证明肌动球蛋白调节因子,包括 Rac1 调节因子,α-PIX 和 RhoA 调节因子,p115-RhoGEF,定位于新生的兴奋性突触,在那里它们优先定位于突触后隔室。这些结果表明,协调的 RhoGTPase 活动是兴奋性突触初始形成的基础,并确定了早期突触发生事件的关键细胞骨架调节剂。
更新日期:2020-10-04
中文翻译:

人类胎儿大脑发育模型中突触发生的细胞骨架调节。
兴奋性突触形成始于中期胎儿妊娠。然而,由于我们无法对胎儿突触发生进行成像,突触的初始形成仍未得到充分研究。人类胎儿脑球体的最新发展为突触形成的这一关键时期提供了途径。使用人类神经元和大脑球体,我们解决了肌动蛋白调节的改变如何影响胎儿大脑发育过程中兴奋性突触的形成。在突触形成之前,抑制 RhoA 激酶 (ROCK) 信号会促进神经突伸长和分支。除了增加神经复杂性之外,ROCK 抑制还会增加沿神经突的突起长度,最终促进人皮质脑球体中兴奋性突触的形成。Rac1 驱动的肌动蛋白聚合的相应增加推动了兴奋性突触发生的增加。使用 STORM 超分辨率显微镜,我们证明肌动球蛋白调节因子,包括 Rac1 调节因子,α-PIX 和 RhoA 调节因子,p115-RhoGEF,定位于新生的兴奋性突触,在那里它们优先定位于突触后隔室。这些结果表明,协调的 RhoGTPase 活动是兴奋性突触初始形成的基础,并确定了早期突触发生事件的关键细胞骨架调节剂。



























 京公网安备 11010802027423号
京公网安备 11010802027423号