当前位置:
X-MOL 学术
›
J. Chem. Thermodyn.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Equilibrium solubility, preferential solvation and solvent effect study of clotrimazole in several aqueous co-solvent solutions
The Journal of Chemical Thermodynamics ( IF 2.6 ) Pub Date : 2020-12-01 , DOI: 10.1016/j.jct.2020.106255 Yanxun Li , Congcong Li , Xiaoqiang Gao , Hekun Lv
The Journal of Chemical Thermodynamics ( IF 2.6 ) Pub Date : 2020-12-01 , DOI: 10.1016/j.jct.2020.106255 Yanxun Li , Congcong Li , Xiaoqiang Gao , Hekun Lv
|
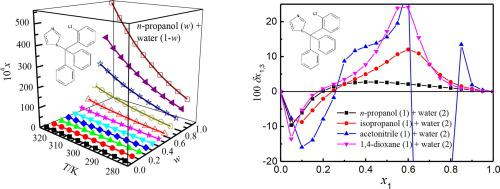
Abstract The equilibrium solubility of clotrimazole in co-solvent mixtures of n-propanol/isopropanol/acetonitrile/1,4-dioxane + water was attained by the saturation shake-flask technique at temperatures between 278.15 K and 323.15 K under ambient pressure of 101.2 kPa. The solubility of clotrimazole in mole fraction scale increased with increasing temperature and mass fractions of n-propanol/isopropanol/acetonitrile/1,4-dioxane. The maximum value was recorded in the neat co-solvent for each mixture studied. The Jouyban-Acree model and van’t Hoff-Jouyban-Acree model were applied to mathematically describe the dependence of clotrimazole solubility upon the co-solvent content and temperature, obtaining the values of relative average deviations and root-mean-square deviations no more than 8.02 × 10−2 and 8.17 × 10−4, individually. KAT-LSER model was implemented to quantitatively acquire evidence about solvent effect upon the variation of clotrimazole solubility magnitudes. The variation in clotrimazole solubility mainly depended upon the Hildebrand solubility parameter and dipolarity-polarizability of solutions in the four aqueous systems studied. Quantitative investigation was carried out for the local mole fractions of n-propanol/isopropanol/acetonitrile/1,4-dioxane and water around solute clotrimazole by using the Inverse Kirkwood–Buff integrals way utilized to the solubility data determined. Clotrimazole was preferentially solvated by n-propanol/isopropanol/acetonitrile/1,4-dioxane for these aqueous co-solvent mixtures in intermediate and n-propanol/isopropanol/acetonitrile/1,4-dioxane-rich proportions.
中文翻译:

克霉唑在几种共溶剂水溶液中的平衡溶解度、优先溶剂化和溶剂效应研究
摘要 克霉唑在正丙醇/异丙醇/乙腈/1,4-二恶烷+水的共溶剂混合物中的平衡溶解度是通过饱和摇瓶技术在278.15 K和323.15 K之间的温度和101.2 kPa的环境压力下获得的。 . 克霉唑在摩尔分数范围内的溶解度随着温度和正丙醇/异丙醇/乙腈/1,4-二恶烷的质量分数的增加而增加。对于所研究的每种混合物,最大值记录在纯共溶剂中。应用Jouyban-Acree模型和van't Hoff-Jouyban-Acree模型从数学上描述了克霉唑溶解度对助溶剂含量和温度的依赖性,得到了相对平均偏差和均方根偏差的数值。分别大于 8.02 × 10−2 和 8.17 × 10−4。实施 KAT-LSER 模型以定量获取关于溶剂对克霉唑溶解度大小变化的影响的证据。克霉唑溶解度的变化主要取决于 Hildebrand 溶解度参数和所研究的四种水性体系中溶液的偶极-极化率。通过使用用于确定溶解度数据的逆柯克伍德-布夫积分方法,对溶质克霉唑周围的正丙醇/异丙醇/乙腈/1,4-二恶烷和水的局部摩尔分数进行了定量研究。克霉唑优先被正丙醇/异丙醇/乙腈/1,4-二氧六环溶剂化,因为这些含水共溶剂混合物呈中间体和正丙醇/异丙醇/乙腈/1,4-二氧六环富集比例。
更新日期:2020-12-01
中文翻译:

克霉唑在几种共溶剂水溶液中的平衡溶解度、优先溶剂化和溶剂效应研究
摘要 克霉唑在正丙醇/异丙醇/乙腈/1,4-二恶烷+水的共溶剂混合物中的平衡溶解度是通过饱和摇瓶技术在278.15 K和323.15 K之间的温度和101.2 kPa的环境压力下获得的。 . 克霉唑在摩尔分数范围内的溶解度随着温度和正丙醇/异丙醇/乙腈/1,4-二恶烷的质量分数的增加而增加。对于所研究的每种混合物,最大值记录在纯共溶剂中。应用Jouyban-Acree模型和van't Hoff-Jouyban-Acree模型从数学上描述了克霉唑溶解度对助溶剂含量和温度的依赖性,得到了相对平均偏差和均方根偏差的数值。分别大于 8.02 × 10−2 和 8.17 × 10−4。实施 KAT-LSER 模型以定量获取关于溶剂对克霉唑溶解度大小变化的影响的证据。克霉唑溶解度的变化主要取决于 Hildebrand 溶解度参数和所研究的四种水性体系中溶液的偶极-极化率。通过使用用于确定溶解度数据的逆柯克伍德-布夫积分方法,对溶质克霉唑周围的正丙醇/异丙醇/乙腈/1,4-二恶烷和水的局部摩尔分数进行了定量研究。克霉唑优先被正丙醇/异丙醇/乙腈/1,4-二氧六环溶剂化,因为这些含水共溶剂混合物呈中间体和正丙醇/异丙醇/乙腈/1,4-二氧六环富集比例。


























 京公网安备 11010802027423号
京公网安备 11010802027423号