Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Sequential changes in histone modifications shape transcriptional responses underlying microglia polarization by glioma.
Glia ( IF 6.2 ) Pub Date : 2020-07-25 , DOI: 10.1002/glia.23887 Marta Maleszewska 1 , Aleksandra Steranka 1 , Magdalena Smiech 1 , Beata Kaza 1 , Paulina Pilanc 1 , Michal Dabrowski 2 , Bozena Kaminska 1
Glia ( IF 6.2 ) Pub Date : 2020-07-25 , DOI: 10.1002/glia.23887 Marta Maleszewska 1 , Aleksandra Steranka 1 , Magdalena Smiech 1 , Beata Kaza 1 , Paulina Pilanc 1 , Michal Dabrowski 2 , Bozena Kaminska 1
Affiliation
|
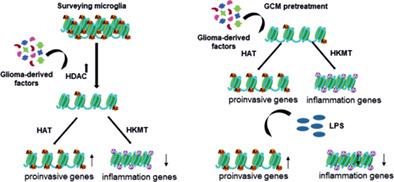
Microglia, resident myeloid cells of the central nervous system (CNS), act as immune sentinels that contribute to maintenance of physiological homeostasis and respond to any perturbation in CNS. Microglia could be polarized by various stimuli to perform dedicated functions and instigate inflammatory or pro‐regenerative responses. Microglia and peripheral macrophages accumulate in glioblastomas (GBMs), malignant brain tumors, but instead of initiating antitumor responses, these cells are polarized to the pro‐invasive and immunosuppressive phenotype which persists for a long time and contributes to a “cold” immune microenvironment of GBMs. Molecular mechanisms underlying this long‐lasting “microglia memory” are unknown. We hypothesized that this state may rely on epigenetic silencing of inflammation‐related genes. In this study, we show that cultured microglia pre‐exposed to glioma‐conditioned medium (GCM) acquire a “transcriptional memory” and display reduced expression of inflammatory genes after re‐stimulation with lipopolysaccharide. Unstimulated microglia have unmethylated DNA and active histone marks at selected gene promoters indicating chromatin accessibility. Adding GCM increases expression and enzymatic activity of histone deacetylases (Hdac), leading to erasure of histone acetylation at tested genes. Later inflammatory genes acquire repressive histone marks (H3K27 trimethylation), which correlates with silencing of their expression. GCM induced genes acquire active histone marks. Hdac inhibitors block GCM‐induced changes of histone modifications and restore microglia ability to initiate effective inflammatory responses. Altogether, we show a scenario of distinct histone modifications underlying polarization of microglia by glioma. We demonstrate contribution of epigenetic mechanisms to glioma‐induced “transcriptional memory” in microglia resulting in the tumor‐supportive phenotype.
中文翻译:

组蛋白修饰的连续变化塑造了胶质瘤引起小胶质细胞极化的转录反应。
小胶质细胞是中枢神经系统 (CNS) 的常驻骨髓细胞,充当免疫哨兵,有助于维持生理稳态并对中枢神经系统的任何扰动作出反应。小胶质细胞可以被各种刺激极化以执行特定的功能并引发炎症或促再生反应。小胶质细胞和外周巨噬细胞在胶质母细胞瘤 (GBM)、恶性脑肿瘤中积聚,但这些细胞并没有启动抗肿瘤反应,而是极化为长期存在的促侵袭和免疫抑制表型,并有助于形成“冷”免疫微环境。 GBM。这种持久的“小胶质细胞记忆”背后的分子机制尚不清楚。我们假设这种状态可能依赖于炎症相关基因的表观遗传沉默。在这项研究中,我们表明,预先暴露于胶质瘤条件培养基 (GCM) 的培养小胶质细胞获得“转录记忆”,并在用脂多糖重新刺激后显示炎症基因的表达减少。未受刺激的小胶质细胞在选定的基因启动子处具有未甲基化的 DNA 和活性组蛋白标记,表明染色质可接近性。添加 GCM 可增加组蛋白去乙酰化酶 (Hdac) 的表达和酶活性,从而消除测试基因上的组蛋白乙酰化。后来的炎症基因获得了抑制性组蛋白标记(H3K27 三甲基化),这与它们的表达沉默相关。GCM 诱导的基因获得活性组蛋白标记。Hdac 抑制剂阻断 GCM 诱导的组蛋白修饰变化并恢复小胶质细胞启动有效炎症反应的能力。共,我们展示了神经胶质瘤对小胶质细胞极化的不同组蛋白修饰的情况。我们证明了表观遗传机制对小胶质细胞中胶质瘤诱导的“转录记忆”的贡献,从而导致肿瘤支持表型。
更新日期:2020-07-25
中文翻译:

组蛋白修饰的连续变化塑造了胶质瘤引起小胶质细胞极化的转录反应。
小胶质细胞是中枢神经系统 (CNS) 的常驻骨髓细胞,充当免疫哨兵,有助于维持生理稳态并对中枢神经系统的任何扰动作出反应。小胶质细胞可以被各种刺激极化以执行特定的功能并引发炎症或促再生反应。小胶质细胞和外周巨噬细胞在胶质母细胞瘤 (GBM)、恶性脑肿瘤中积聚,但这些细胞并没有启动抗肿瘤反应,而是极化为长期存在的促侵袭和免疫抑制表型,并有助于形成“冷”免疫微环境。 GBM。这种持久的“小胶质细胞记忆”背后的分子机制尚不清楚。我们假设这种状态可能依赖于炎症相关基因的表观遗传沉默。在这项研究中,我们表明,预先暴露于胶质瘤条件培养基 (GCM) 的培养小胶质细胞获得“转录记忆”,并在用脂多糖重新刺激后显示炎症基因的表达减少。未受刺激的小胶质细胞在选定的基因启动子处具有未甲基化的 DNA 和活性组蛋白标记,表明染色质可接近性。添加 GCM 可增加组蛋白去乙酰化酶 (Hdac) 的表达和酶活性,从而消除测试基因上的组蛋白乙酰化。后来的炎症基因获得了抑制性组蛋白标记(H3K27 三甲基化),这与它们的表达沉默相关。GCM 诱导的基因获得活性组蛋白标记。Hdac 抑制剂阻断 GCM 诱导的组蛋白修饰变化并恢复小胶质细胞启动有效炎症反应的能力。共,我们展示了神经胶质瘤对小胶质细胞极化的不同组蛋白修饰的情况。我们证明了表观遗传机制对小胶质细胞中胶质瘤诱导的“转录记忆”的贡献,从而导致肿瘤支持表型。


























 京公网安备 11010802027423号
京公网安备 11010802027423号