当前位置:
X-MOL 学术
›
Biomacromolecules
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Disordered Filaments Mediate the Fibrillogenesis of Type I Collagen in Solution.
Biomacromolecules ( IF 6.2 ) Pub Date : 2020-07-24 , DOI: 10.1021/acs.biomac.0c00667 Andrew R McCluskey 1 , Kennes S W Hung 1 , Bartosz Marzec 1 , Julien O Sindt 2 , Nico A J M Sommerdijk 3 , Philip J Camp 1 , Fabio Nudelman 1
Biomacromolecules ( IF 6.2 ) Pub Date : 2020-07-24 , DOI: 10.1021/acs.biomac.0c00667 Andrew R McCluskey 1 , Kennes S W Hung 1 , Bartosz Marzec 1 , Julien O Sindt 2 , Nico A J M Sommerdijk 3 , Philip J Camp 1 , Fabio Nudelman 1
Affiliation
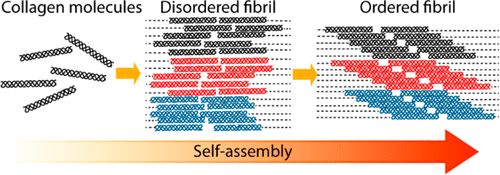
|
Collagen type I is one of the major structural proteins in mammals, providing tissues such as cornea, tendon, bone, skin, and dentin with mechanical stability, strength, and toughness. Collagen fibrils are composed of collagen molecules arranged in a quarter-stagger array that gives rise to a periodicity of 67 nm along the fibril axis, with a 30 nm overlap zone and a 37 nm gap zone. The formation of such highly organized fibrils is a self-assembly process where electrostatic and hydrophobic interactions play a critical role in determining the staggering of the molecules with 67 nm periodicity. While collagen self-assembly has been extensively studied, not much is known about the mechanism, and in particular, the nature of the nuclei that initially form, the different stages of the aggregation process, and how the organization of the molecules into fibrils arises. By combining time-resolved cryo-transmission electron microscopy with molecular dynamics simulations, we show that collagen assembly is a multistep process in which the molecules first form filaments which self-organize into fibrils with a disordered structure. The appearance of the D-band periodicity is gradual and starts with the alignment of adjacent filaments at the N-terminal end of the molecules, first leading to bands with a periodicity of 67 nm and then to the formation of gap and overlap regions.
中文翻译:

无序的细丝介导溶液中I型胶原的原纤维形成。
I型胶原蛋白是哺乳动物的主要结构蛋白之一,可为角膜,腱,骨骼,皮肤和牙本质等组织提供机械稳定性,强度和韧性。胶原蛋白原纤维由以四分之一交错排列的胶原蛋白分子组成,该胶原蛋白分子沿着原纤维轴产生67 nm的周期性,具有30 nm的重叠区和37 nm的间隙区。这种高度组织化的原纤维的形成是自组装过程,其中静电和疏水相互作用在确定具有67 nm周期性的分子错开中起关键作用。尽管已经对胶原蛋白的自组装进行了广泛的研究,但对其机理,尤其是最初形成的核的性质,聚集过程的不同阶段,以及分子如何组织成原纤维。通过将时间分辨的低温透射电子显微镜与分子动力学模拟相结合,我们表明胶原蛋白组装是一个多步过程,其中分子首先形成细丝,这些细丝会自组织成具有无序结构的原纤维。D波段周期性的出现是渐进的,并从分子N端的相邻细丝对齐开始,首先导致具有67 nm周期性的波段,然后形成间隙和重叠区域。
更新日期:2020-09-14
中文翻译:

无序的细丝介导溶液中I型胶原的原纤维形成。
I型胶原蛋白是哺乳动物的主要结构蛋白之一,可为角膜,腱,骨骼,皮肤和牙本质等组织提供机械稳定性,强度和韧性。胶原蛋白原纤维由以四分之一交错排列的胶原蛋白分子组成,该胶原蛋白分子沿着原纤维轴产生67 nm的周期性,具有30 nm的重叠区和37 nm的间隙区。这种高度组织化的原纤维的形成是自组装过程,其中静电和疏水相互作用在确定具有67 nm周期性的分子错开中起关键作用。尽管已经对胶原蛋白的自组装进行了广泛的研究,但对其机理,尤其是最初形成的核的性质,聚集过程的不同阶段,以及分子如何组织成原纤维。通过将时间分辨的低温透射电子显微镜与分子动力学模拟相结合,我们表明胶原蛋白组装是一个多步过程,其中分子首先形成细丝,这些细丝会自组织成具有无序结构的原纤维。D波段周期性的出现是渐进的,并从分子N端的相邻细丝对齐开始,首先导致具有67 nm周期性的波段,然后形成间隙和重叠区域。


























 京公网安备 11010802027423号
京公网安备 11010802027423号