当前位置:
X-MOL 学术
›
Z. Anorg. Allg. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Room Temperature Synthesis of New Thiostannates by Slow Interdiffusion of Different Solvents
Zeitschrift für anorganische und allgemeine Chemie ( IF 1.4 ) Pub Date : 2020-07-09 , DOI: 10.1002/zaac.202000199 Assma Benkada 1 , Christian Näther 1 , Wolfgang Bensch 1
Zeitschrift für anorganische und allgemeine Chemie ( IF 1.4 ) Pub Date : 2020-07-09 , DOI: 10.1002/zaac.202000199 Assma Benkada 1 , Christian Näther 1 , Wolfgang Bensch 1
Affiliation
|
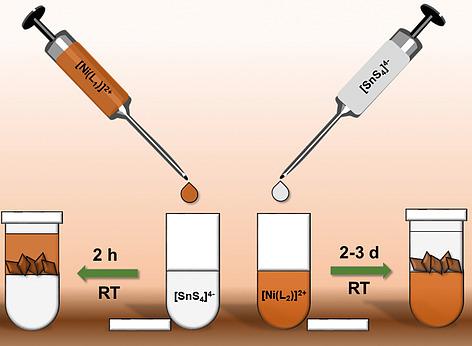
The new compounds [Ni(L1)][Ni(L1)Sn2S6]n·2H2O (I) and [Ni(L2)]2[Sn2S6]·4H2O (II) containing the macrocyclic ligands L1 (L1 = 1,8‐dimethyl‐1,3,6,8,10,13‐hexaazacyclotetradecane) and L2 (L2 = 1,8‐diethyl‐1,3,6,8,10,13‐hexaazacyclotetradecane) were prepared at room temperature by overlaying an aqueous solution of Na4SnS4·14H2O with the [Ni(L1)](ClO4)2 complex dissolved in CH3CN (I) or by overlaying a solution of the [Ni(L2)](ClO4)2 complex dissolved in DMSO with an aqueous solution of Na4SnS4·14H2O (II). The slow interdiffusion of the two solvents guarantees supersaturation in the interface region of the solvents so that crystallization of the compounds occurs. In the structure of I one Ni2+ cation has bonds to S2– anions of the thiostannate anion thus generating chains along [100]. This cation is in an octahedral environment of four N atoms of L1 and two S atoms of the [Sn2S6]4– anion. The second [Ni(L1)]2+ complex exhibits a square‐planar coordination geometry. These [Ni(L1)]2+ complexes and water molecules are located between the chains. In the structure of II isolated [Sn2S6]4– anions and [Ni(L2)]2+ cations are observed. The Ni2+ cations are fourfold coordinated by N atoms of the L2 ligand and feature also a square planar environment.
中文翻译:

室温慢扩散不同溶剂合成新的硫代锡酸盐
新化合物[Ni(L 1)] [Ni(L 1)Sn 2 S 6 ] n · 2H 2 O(I)和[Ni(L 2)] 2 [Sn 2 S 6 ] · 4H 2 O(II)包含大环配体L 1(L 1 = 1,8-二甲基-1,3,6,8,10,13-六氮杂环十四烷)和L 2(L 2 = 1,8-二乙基-1,3,6,在室温下通过覆盖Na 4 SnS 4水溶液制备8,10,13-六氮杂环十四烷)·用[Ni(L 1)](ClO 4)2络合物溶于CH 3 CN(I)或通过将[Ni(L 2)](ClO 4)2络合物溶于DMSO的溶液覆盖14H 2 O用Na 4 SnS 4 · 14H 2 O(II)的水溶液。两种溶剂的缓慢相互扩散确保了溶剂界面区域中的过饱和,从而使化合物发生结晶。在I的结构中,一个Ni 2+阳离子与S 2–硫锡酸根阴离子的阴离子因此沿着[100]生成链。该阳离子处于L 1的四个N原子和[Sn 2 S 6 ] 4–阴离子的两个S原子的八面体环境中。第二个[Ni(L 1)] 2+络合物具有正方形-平面的配位几何形状。这些[Ni(L 1)] 2+络合物和水分子位于链之间。在II的结构中,观察到了[Sn 2 S 6 ] 4–阴离子和[Ni(L 2)] 2+阳离子。镍2+阳离子由L 2配体的N个原子四重配位,并且还具有正方形平面环境。
更新日期:2020-07-09
中文翻译:

室温慢扩散不同溶剂合成新的硫代锡酸盐
新化合物[Ni(L 1)] [Ni(L 1)Sn 2 S 6 ] n · 2H 2 O(I)和[Ni(L 2)] 2 [Sn 2 S 6 ] · 4H 2 O(II)包含大环配体L 1(L 1 = 1,8-二甲基-1,3,6,8,10,13-六氮杂环十四烷)和L 2(L 2 = 1,8-二乙基-1,3,6,在室温下通过覆盖Na 4 SnS 4水溶液制备8,10,13-六氮杂环十四烷)·用[Ni(L 1)](ClO 4)2络合物溶于CH 3 CN(I)或通过将[Ni(L 2)](ClO 4)2络合物溶于DMSO的溶液覆盖14H 2 O用Na 4 SnS 4 · 14H 2 O(II)的水溶液。两种溶剂的缓慢相互扩散确保了溶剂界面区域中的过饱和,从而使化合物发生结晶。在I的结构中,一个Ni 2+阳离子与S 2–硫锡酸根阴离子的阴离子因此沿着[100]生成链。该阳离子处于L 1的四个N原子和[Sn 2 S 6 ] 4–阴离子的两个S原子的八面体环境中。第二个[Ni(L 1)] 2+络合物具有正方形-平面的配位几何形状。这些[Ni(L 1)] 2+络合物和水分子位于链之间。在II的结构中,观察到了[Sn 2 S 6 ] 4–阴离子和[Ni(L 2)] 2+阳离子。镍2+阳离子由L 2配体的N个原子四重配位,并且还具有正方形平面环境。



























 京公网安备 11010802027423号
京公网安备 11010802027423号