当前位置:
X-MOL 学术
›
Z. Anorg. Allg. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
On the Carbonate Formation in Thermally Stressed Hydroxysodalite – Some Facts to Notice for SOD Application in Separation Processes
Zeitschrift für anorganische und allgemeine Chemie ( IF 1.4 ) Pub Date : 2020-07-02 , DOI: 10.1002/zaac.202000051 Irma Peschke 1 , Hagen Hildebrand 1 , Robert Pioch 1 , Josef-Christian Buhl 1
Zeitschrift für anorganische und allgemeine Chemie ( IF 1.4 ) Pub Date : 2020-07-02 , DOI: 10.1002/zaac.202000051 Irma Peschke 1 , Hagen Hildebrand 1 , Robert Pioch 1 , Josef-Christian Buhl 1
Affiliation
|
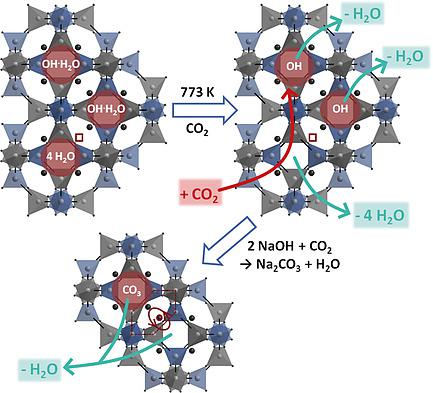
Basic sodalite (hydroxysodalite) was synthesized from two Si‐Al sources: (1) kaolin to obtain |Na7.5(OH)1.5(H2O)3.5|[AlSiO4]6 sodalites (SOD) with small crystals (< 0.5 μm) and (2) a mixture of cristobalite and corundum (CC) to obtain larger microcrystals (1–5 μm) with ideal composition |Na8(OH)2(H2O)2|[AlSiO4]6. Both SOD were exposed to thermal stress by long‐time heating at 773 K under open conditions, in N2 and CO2 atmosphere and in presence of a NaOH‐Na2CO3 melt. The crystals obtained from kaolin were dehydrated and developed remarkable degrees of carbonate cage fillings already under open conditions. The large microcrystals obtained from CC exhibit this effect only at very low scale even after long‐time heating in CO2 atmosphere. Whereas heating in presence of the melt showed no effect, investigations in CO2 clearly indicate an intra‐cage reaction between CO2 and the enclathrated [Na4OH]3+ ions as the carbonate generating mechanism instead of destruction of hydroxysodalite followed by recrystallization. A model is proposed, in which cage fillings [Na3□]3+ with a vacancy in the Na tetrahedron known from dehydrated hydrosodalites |Na6|[AlSiO4]6 are required to induce the intra‐cage reaction of hydroxysodalite. As those fillings only occur in a noticeable number in dehydrated hydroxysodalite obtained from kaolin, the large extent of the carbonate formation inside this sample becomes obvious. The results are significant for future improvement of hydroxysodalite membranes.
中文翻译:

关于热应力羟基钠钙石中碳酸盐的形成——SOD在分离过程中应注意的一些事实
碱性方钠石(羟基钠钙石)是由两种Si-Al来源合成的:(1)高岭土获得| Na 7.5(OH)1.5(H 2 O)3.5 | [AlSiO 4 ] 6方钠石(SOD),具有小晶体(<0.5μm) )和(2)方石英和刚玉(CC)的混合物,以获得具有理想组成| Na 8(OH)2(H 2 O)2 | [AlSiO 4 ] 6的较大微晶(1-5μm)。在开放条件下,N 2和CO 2气氛中以及存在NaOH-Na 2的条件下,在773 K下长时间加热,两种SOD都暴露于热应力下。CO 3熔体。从高岭土获得的晶体已经脱水,并且已经在开放条件下形成了显着程度的碳酸盐笼状填充物。即使在CO 2气氛中长时间加热后,从CC中获得的大型微晶也仅在很小的规模上显示出这种效果。尽管在熔体存在下加热没有影响,但对CO 2的研究清楚地表明,CO 2和包裹的[Na 4 OH] 3+离子之间的笼内反应是碳酸盐生成的机理,而不是破坏羟基钠钙石,然后进行重结晶。提出了一种模型,其中笼式填充物[Na 3 □] 3+脱水水苏打中已知的Na四面体中存在空位| Na 6 | [AlSiO 4 ] 6才能引起羟基苏打的笼内反应。由于这些填充物仅在从高岭土中获得的脱水羟基苏打石中显着出现,因此该样品内部大量碳酸盐形成变得明显。该结果对于羟基钠钙石膜的未来改进具有重要意义。
更新日期:2020-07-02
中文翻译:

关于热应力羟基钠钙石中碳酸盐的形成——SOD在分离过程中应注意的一些事实
碱性方钠石(羟基钠钙石)是由两种Si-Al来源合成的:(1)高岭土获得| Na 7.5(OH)1.5(H 2 O)3.5 | [AlSiO 4 ] 6方钠石(SOD),具有小晶体(<0.5μm) )和(2)方石英和刚玉(CC)的混合物,以获得具有理想组成| Na 8(OH)2(H 2 O)2 | [AlSiO 4 ] 6的较大微晶(1-5μm)。在开放条件下,N 2和CO 2气氛中以及存在NaOH-Na 2的条件下,在773 K下长时间加热,两种SOD都暴露于热应力下。CO 3熔体。从高岭土获得的晶体已经脱水,并且已经在开放条件下形成了显着程度的碳酸盐笼状填充物。即使在CO 2气氛中长时间加热后,从CC中获得的大型微晶也仅在很小的规模上显示出这种效果。尽管在熔体存在下加热没有影响,但对CO 2的研究清楚地表明,CO 2和包裹的[Na 4 OH] 3+离子之间的笼内反应是碳酸盐生成的机理,而不是破坏羟基钠钙石,然后进行重结晶。提出了一种模型,其中笼式填充物[Na 3 □] 3+脱水水苏打中已知的Na四面体中存在空位| Na 6 | [AlSiO 4 ] 6才能引起羟基苏打的笼内反应。由于这些填充物仅在从高岭土中获得的脱水羟基苏打石中显着出现,因此该样品内部大量碳酸盐形成变得明显。该结果对于羟基钠钙石膜的未来改进具有重要意义。



























 京公网安备 11010802027423号
京公网安备 11010802027423号