当前位置:
X-MOL 学术
›
Pept. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Constrained Glu‐Gly and Gln‐Gly dipeptide surrogates from γ‐substituted α‐amino‐δ‐lactam synthesis
Peptide Science ( IF 2.4 ) Pub Date : 2020-01-14 , DOI: 10.1002/pep2.24149 Ramakotaiah Mulamreddy 1 , William D. Lubell 1
Peptide Science ( IF 2.4 ) Pub Date : 2020-01-14 , DOI: 10.1002/pep2.24149 Ramakotaiah Mulamreddy 1 , William D. Lubell 1
Affiliation
|
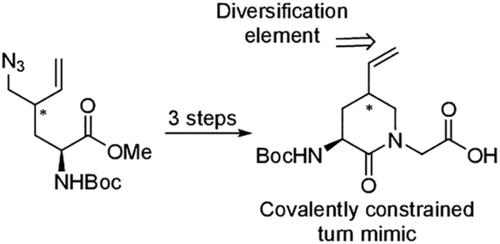
Conformationally rigid α‐amino‐δ‐lactam surrogates of Glu‐Gly and Gln‐Gly dipeptides have been synthesized from α‐amino‐γ‐vinyl‐δ‐lactam precursors. A reductive cyclization protocol from the respective (4R)‐ and (4S)‐2‐N‐(Boc)amino‐4‐(azidomethyl)hexenoates gave δ‐lactams, which were converted to the corresponding dipeptide surrogates by N‐alkylation with methyl bromoacetate. The utility of these α‐amino‐γ‐vinyl‐δ‐lactam building blocks was demonstrated by olefin oxidation and peptide coupling to prepare constrained Glu and Gln residues.
中文翻译:

γ-取代的α-氨基-δ-内酰胺合成中受约束的Glu-Gly和Gln-Gly二肽替代物
Glu-Gly和Gln-Gly二肽的构形刚性α-氨基-δ-内酰胺替代物是由α-氨基-γ-乙烯基-δ-内酰胺前体合成的。(4 R)-和(4 S)-2- N-(Boc)氨基-4-(叠氮基甲基)己酸根的还原环化反应产生δ-内酰胺,通过N-烷基化将其转化为相应的二肽替代物用溴乙酸甲酯。这些α-氨基-γ-乙烯基-δ-内酰胺结构单元的用途通过烯烃氧化和肽偶联制备受限的Glu和Gln残基得到了证明。
更新日期:2020-01-14
中文翻译:

γ-取代的α-氨基-δ-内酰胺合成中受约束的Glu-Gly和Gln-Gly二肽替代物
Glu-Gly和Gln-Gly二肽的构形刚性α-氨基-δ-内酰胺替代物是由α-氨基-γ-乙烯基-δ-内酰胺前体合成的。(4 R)-和(4 S)-2- N-(Boc)氨基-4-(叠氮基甲基)己酸根的还原环化反应产生δ-内酰胺,通过N-烷基化将其转化为相应的二肽替代物用溴乙酸甲酯。这些α-氨基-γ-乙烯基-δ-内酰胺结构单元的用途通过烯烃氧化和肽偶联制备受限的Glu和Gln残基得到了证明。



























 京公网安备 11010802027423号
京公网安备 11010802027423号