当前位置:
X-MOL 学术
›
J. Leukoc. Biol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Drug target validation in primary human natural killer cells using CRISPR RNP
Journal of Leukocyte Biology ( IF 5.5 ) Pub Date : 2020-07-17 , DOI: 10.1002/jlb.2ma0620-074r Jai Rautela 1, 2 , Elliot Surgenor 1 , Nicholas D. Huntington 1, 2
Journal of Leukocyte Biology ( IF 5.5 ) Pub Date : 2020-07-17 , DOI: 10.1002/jlb.2ma0620-074r Jai Rautela 1, 2 , Elliot Surgenor 1 , Nicholas D. Huntington 1, 2
Affiliation
|
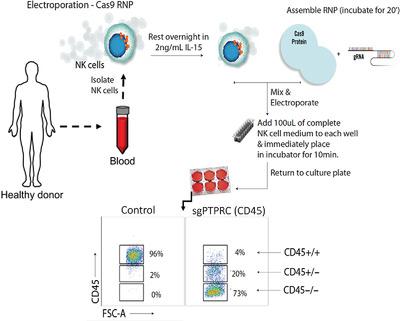
The ability to genetically modify CD8 T cells using viral gene delivery has facilitated the development of next generation of cancer immunotherapies such as chimeric Ag receptor (CAR) T cells engineered to specifically kill tumor cells. Development of immunotherapies targeting NK cells have stalled in part by their resistance to traditional viral gene delivery systems. Here, an efficient approach is described to genetically edit human NK cells by electroporation and CRISPR‐Cas9 ribonucleoprotein (RNP) complexes. Electroporation pulse codes and buffer optimization for protein uptake by human NK cells and viability, and the efficiency of this approach over other methods are detailed. To highlight the transformative step this technique will have for NK cell immunotherapy drug discovery, NCR1 and CISH are deleted in primary human NK cells and murine findings are validated on their key roles in regulating NK cell antitumor function.
中文翻译:

使用CRISPR RNP在主要人类自然杀伤细胞中进行药物靶标验证
利用病毒基因传递来遗传修饰CD8 T细胞的能力促进了下一代癌症免疫疗法的发展,例如经过工程改造以特异性杀死肿瘤细胞的嵌合Ag受体(CAR)T细胞。靶向NK细胞的免疫疗法的发展由于其对传统病毒基因传递系统的抗性而部分停止。在这里,描述了一种有效的方法来通过电穿孔和CRISPR‐Cas9核糖核蛋白(RNP)复合物对人类NK细胞进行遗传编辑。详细介绍了用于人NK细胞蛋白质摄取和生存力的电穿孔脉冲编码和缓冲液优化,以及该方法相对于其他方法的效率。为了突出这项技术在NK细胞免疫疗法药物发现中所具有的变革性步骤,
更新日期:2020-07-17
中文翻译:

使用CRISPR RNP在主要人类自然杀伤细胞中进行药物靶标验证
利用病毒基因传递来遗传修饰CD8 T细胞的能力促进了下一代癌症免疫疗法的发展,例如经过工程改造以特异性杀死肿瘤细胞的嵌合Ag受体(CAR)T细胞。靶向NK细胞的免疫疗法的发展由于其对传统病毒基因传递系统的抗性而部分停止。在这里,描述了一种有效的方法来通过电穿孔和CRISPR‐Cas9核糖核蛋白(RNP)复合物对人类NK细胞进行遗传编辑。详细介绍了用于人NK细胞蛋白质摄取和生存力的电穿孔脉冲编码和缓冲液优化,以及该方法相对于其他方法的效率。为了突出这项技术在NK细胞免疫疗法药物发现中所具有的变革性步骤,



























 京公网安备 11010802027423号
京公网安备 11010802027423号