当前位置:
X-MOL 学术
›
Immun. Inflamm. Dis.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Necroptotic-susceptible dendritic cells exhibit enhanced antitumor activities in mice.
Immunity, Inflammation and Disease ( IF 2.493 ) Pub Date : 2020-07-14 , DOI: 10.1002/iid3.330 Zhanran Zhao 1 , Guangzhi Zhang 1 , Yuefang Sun 1 , Astar Winoto 1
Immunity, Inflammation and Disease ( IF 2.493 ) Pub Date : 2020-07-14 , DOI: 10.1002/iid3.330 Zhanran Zhao 1 , Guangzhi Zhang 1 , Yuefang Sun 1 , Astar Winoto 1
Affiliation
|
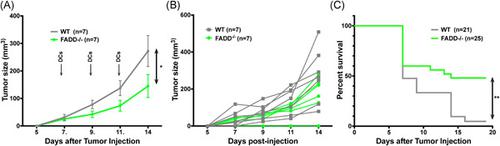
Priming of tumor‐specific T cells is a key to antitumor immune response and inflammation, in turn, is crucial for proper T‐cell activation. As antigen‐presenting cells can activate T cells, dendritic cells (DCs) loaded with tumor antigens have been used as immunotherapeutics against certain cancer in humans but their efficacy is modest. Necroptosis is a form of programmed cell death that results in the release of inflammatory contents. We previously generated mice with DC deficiency in a negative regulator of necroptosis, Fas‐associated death domain (FADD), and found that these mice suffer from systemic inflammation due to necroptotic DCs. We hypothesize that FADD‐deficient DCs could serve as a better vaccine than wild‐type (WT) DCs against tumors.
中文翻译:

坏死性凋亡敏感的树突状细胞在小鼠中表现出增强的抗肿瘤活性。
肿瘤特异性 T 细胞的启动是抗肿瘤免疫反应和炎症的关键,反过来,对于 T 细胞的正确激活也至关重要。由于抗原呈递细胞可以激活 T 细胞,负载肿瘤抗原的树突状细胞 (DC) 已被用作针对人类某些癌症的免疫疗法,但其疗效有限。坏死性凋亡是一种程序性细胞死亡形式,导致炎症内容物的释放。我们之前生成了坏死性凋亡负调节因子 Fas 相关死亡结构域 (FADD) 中 DC 缺陷的小鼠,并发现这些小鼠因坏死性 DC 而患有全身炎症。我们假设 FADD 缺陷的 DC 可以作为比野生型 (WT) DC 更好的肿瘤疫苗。
更新日期:2020-08-10
中文翻译:

坏死性凋亡敏感的树突状细胞在小鼠中表现出增强的抗肿瘤活性。
肿瘤特异性 T 细胞的启动是抗肿瘤免疫反应和炎症的关键,反过来,对于 T 细胞的正确激活也至关重要。由于抗原呈递细胞可以激活 T 细胞,负载肿瘤抗原的树突状细胞 (DC) 已被用作针对人类某些癌症的免疫疗法,但其疗效有限。坏死性凋亡是一种程序性细胞死亡形式,导致炎症内容物的释放。我们之前生成了坏死性凋亡负调节因子 Fas 相关死亡结构域 (FADD) 中 DC 缺陷的小鼠,并发现这些小鼠因坏死性 DC 而患有全身炎症。我们假设 FADD 缺陷的 DC 可以作为比野生型 (WT) DC 更好的肿瘤疫苗。


























 京公网安备 11010802027423号
京公网安备 11010802027423号