Journal of Environmental Chemical Engineering ( IF 7.7 ) Pub Date : 2020-05-21 , DOI: 10.1016/j.jece.2020.104072 Weng Fu , Guozhao Ji , Huihuang Chen , Siyuan Yang , Hong Yang , Bao Guo , Zhiqiang Huang
|
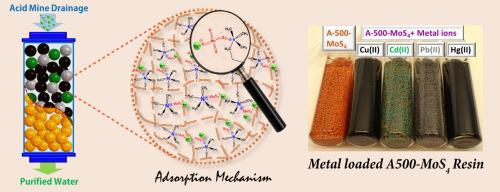
Acid mine drainage (AMD) is an acidic effluent containing many toxic heavy metal ions (e.g. Cu2+, Cd2+, Hg2+) in mining industry, leading to serious environmental issues such as natural soil and aquatic pollution that threats the whole ecosystem and biosafety. Conventional neutralization and precipitation process cannot effectively remove heavy metal ions due to the risk of secondary contamination. Herein, we report an engineered amorphous molybdenum sulphide composite for selective removal of heavy metal ions from other co-existing ions in AMD solution. This composite was prepared by a facile ion exchange reaction, in which tetrathiomolybdate (MoS42-) anions are firmly bonded to strong anion exchange resins by replacing chloride ions. The obtained composite was used to remove heavy metal ions from both synthetic and authentic AMD solution. The results of batch and fixed-bed column tests suggest high selectivity towards heavy metals, fast adsorption kinetics, good reusability and excellent adsorption capacities in the order of Hg(II)>>Pb(II)>Cu(II)>Cd(II). The adsorption data are fitted well by Langmuir model, indicating the single-layer adsorption mechanism. The theoretical adsorption capacities calculated by Langmuir model are 259.0 mg/g for Cu(II), 204.1 mg/g for Cd(II), 495.0 mg/g for Pb(II) and 1538.4 mg/g for Hg(II). The interaction between metal ions (Cu, Cd, Pb, Hg) and MoS42- anions are demonstrated by the formation of Mo-S-metal (Cu, Cd, Pb, Hg) bonding (the red shift of S2p peak in XPS spectra). Our results support the potential practical application of this new material for scavenging heavy metal ions in AMD wastewater.
中文翻译:

工程阴离子树脂基无定形硫化钼复合材料用于处理地雷酸性矿山
酸性矿山排水(AMD)是一种酸性废水,在采矿业中含有许多有毒的重金属离子(例如Cu 2 +,Cd 2 +,Hg 2+),从而导致严重的环境问题,例如天然土壤和水生污染,威胁着整个环境生态系统和生物安全。由于存在二次污染的风险,传统的中和和沉淀过程无法有效去除重金属离子。本文中,我们报道了一种工程非晶态硫化钼复合材料,用于从AMD溶液中其他共存离子中选择性去除重金属离子。该复合材料是通过容易的离子交换反应制备的,其中四硫代钼酸盐(MoS 4 2-)阴离子通过取代氯离子与强阴离子交换树脂牢固结合。所获得的复合材料用于从合成的和真实的AMD溶液中去除重金属离子。分批和固定床柱测试的结果表明,Hg(II)>> Pb(II)> Cu(II)> Cd(II)的顺序具有对重金属的高选择性,快速的吸附动力学,良好的可重复使用性和出色的吸附能力。 )。吸附数据与Langmuir模型拟合得很好,表明了单层吸附机理。由Langmuir模型计算得出的理论吸附容量对Cu(II)为259.0 mg / g,对Cd(II)为204.1 mg / g,对Pb(II)为495.0 mg / g和对Hg(II)1538.4 mg / g。金属离子(Cu,Cd,Pb,Hg)与MoS 4 2-之间的相互作用通过形成Mo-S-金属(Cu,Cd,Pb,Hg)键(XPS光谱中S2p峰的红移)可以证明阴离子。我们的结果支持了这种新材料在AMD废水中清除重金属离子的潜在实际应用。


























 京公网安备 11010802027423号
京公网安备 11010802027423号