Differentiation ( IF 2.9 ) Pub Date : 2020-07-17 , DOI: 10.1016/j.diff.2020.06.003 Sakhavat Abolhasani 1 , Masoumeh Rajabibazl 1 , Mohammad-Mehdi Khani 2 , Azim Parandakh 3 , Reyhaneh Hoseinpoor 4
|
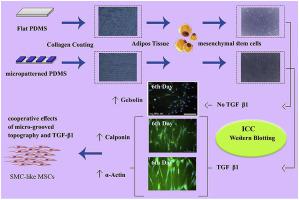
Cell morphological changes induced by micro-grooved topography have been shown to be an important regulator of smooth muscle (SM) differentiation of mesenchymal stem cells (MSCs). In addition to the micro-grooved topography, transforming growth factor-β1 (TGF-β1) can also modulate MSCs differentiation towards smooth muscle cells (SMCs) through alterations in cell morphological characteristics. Thus, it can be hypothesized that substrate topography and TGF-β1 may interact to facilitate differentiation of MSCs into SMCs. In this study, we investigated the time-course cooperative effects of substrate topography and TGF-β1 in the regulation of SM differentiation of human MSCs. Western blotting, followed by image analysis, was performed to assess the protein expression of α-actin, h1-calponin and gelsolin. Three-way analysis of variance was employed to investigate the main effect of each independent variable, i.e. TGF-β1 conditioning, substrate topography and culture time, along with the interactions of these variables. Each of TGF-β1, substrate topography and culture time significantly affected the protein expression of α-actin, h1-calponin and gelsolin. Overall, TGF-β1 conditioning of the cells and culturing the cells on the micro-grooved substrate resulted in greater protein expression of α-actin and h1-calponin, and lesser protein expression of gelsolin. In addition to the isolated effects of the variables, treatment type interacted with substrate topography and culture time to regulate the expression of the above-mentioned proteins. This study indicated the feasibility of promoting SM differentiation of human MSCs by simultaneous recruitment of micro-grooved topography and TGF-β1. The findings could be of assistance when effective utilization of chemo-physical cues is needed to achieve functional SMC-like MSCs in vitro.
中文翻译:

微槽形貌与TGF-β1对间充质干细胞血管平滑肌细胞收缩蛋白表达的协同作用。
微槽形貌诱导的细胞形态变化已被证明是间充质干细胞 (MSC) 平滑肌 (SM) 分化的重要调节因子。除了微槽形貌,转化生长因子-β 1 (TGF-β 1 ) 还可以通过细胞形态特征的改变来调节 MSCs 向平滑肌细胞 (SMCs) 的分化。因此,可以假设衬底地形和TGF-β 1可相互作用以促进MSC的分化为平滑肌细胞。在这项研究中,我们调查衬底地形和TGF-β的时间过程协同效应1在调节人MSCs的SM分化中。进行蛋白质印迹,然后进行图像分析,以评估 α-肌动蛋白、h1-钙调蛋白和凝溶胶蛋白的蛋白质表达。方差的三通分析来探讨每个独立变量,即TGF-β的主要作用1空调,基板地形和培养时间,与这些变量的相互作用沿。TGF-β 1、底物形貌和培养时间均显着影响α-肌动蛋白、h1-钙调蛋白和凝溶胶蛋白的蛋白表达。总体而言,TGF-β 1细胞的调理和在微槽基质上培养细胞导致 α-肌动蛋白和 h1-钙调蛋白的蛋白质表达更高,而凝溶胶蛋白的蛋白质表达更少。除了这些变量的孤立效应外,处理类型还与底物地形和培养时间相互作用,以调节上述蛋白质的表达。该研究表明通过同时募集微槽形貌和 TGF-β 1促进人 MSC 的 SM 分化的可行性。当需要有效利用化学物理线索来实现体外功能性 SMC 样 MSC 时,这些发现可能会有所帮助。



























 京公网安备 11010802027423号
京公网安备 11010802027423号