当前位置:
X-MOL 学术
›
Catal. Sci. Technol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
The effect of strong metal–support interaction (SMSI) on Pt–Ti/SiO2 and Pt–Nb/SiO2 catalysts for propane dehydrogenation
Catalysis Science & Technology ( IF 5 ) Pub Date : 2020-07-23 , DOI: 10.1039/d0cy00897d Johnny Zhu Chen 1, 2, 3 , Junxian Gao 1, 2, 3 , Paige R. Probus 1, 2, 3 , Wei Liu 4, 5, 6, 7 , Xianli Wu 1, 2, 3, 8, 9 , Evan C. Wegener 3, 10, 11, 12 , A. Jeremy Kropf 3, 10, 11, 12 , Dmitry Zemlyanov 2, 3, 13, 14 , Guanghui Zhang 15, 16, 17, 18, 19 , Xin Yang 1, 2, 3, 17, 20 , Jeffrey T. Miller 1, 2, 3
Catalysis Science & Technology ( IF 5 ) Pub Date : 2020-07-23 , DOI: 10.1039/d0cy00897d Johnny Zhu Chen 1, 2, 3 , Junxian Gao 1, 2, 3 , Paige R. Probus 1, 2, 3 , Wei Liu 4, 5, 6, 7 , Xianli Wu 1, 2, 3, 8, 9 , Evan C. Wegener 3, 10, 11, 12 , A. Jeremy Kropf 3, 10, 11, 12 , Dmitry Zemlyanov 2, 3, 13, 14 , Guanghui Zhang 15, 16, 17, 18, 19 , Xin Yang 1, 2, 3, 17, 20 , Jeffrey T. Miller 1, 2, 3
Affiliation
|
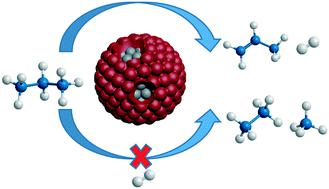
In this study, we show how strong metal–support interaction (SMSI) oxides in Pt–Nb/SiO2 and Pt–Ti/SiO2 affect the electronic, geometric and catalytic properties for propane dehydrogenation. Transmission electron microscopy (TEM), CO chemisorption, and decrease in the catalytic rates per gram Pt confirm that the Pt nanoparticles were partially covered by the SMSI oxides. X-ray absorption near edge structure (XANES), in situ X-ray photoelectron spectroscopy (XPS), and resonant inelastic X-ray scattering (RIXS) showed little change in the energy of Pt valence orbitals upon interaction with SMSI oxides. The catalytic activity per mol of Pt for ethylene hydrogenation and propane dehydrogenation was lower due to fewer exposed Pt sites, while turnover rates were similar. The SMSI oxides, however, significantly increase the propylene selectivity for the latter reaction compared to Pt/SiO2. In the SMSI catalysts, the higher olefin selectivity is suggested to be due to the smaller exposed Pt ensemble sites, which result in suppression of the alkane hydrogenolysis reaction; while the exposed atoms remain active for dehydrogenation.
中文翻译:

强金属-载体相互作用(SMSI)对丙烷脱氢用Pt-Ti / SiO2和Pt-Nb / SiO2催化剂的影响
在这项研究中,我们显示了Pt–Nb / SiO 2和Pt–Ti / SiO 2中的强金属-载体相互作用(SMSI)氧化物如何影响丙烷脱氢的电子,几何和催化性能。透射电子显微镜(TEM),CO化学吸附以及每克Pt催化速率的降低证实了Pt纳米颗粒被SMSI氧化物部分覆盖。原位边缘结构(XANES)附近的X射线吸收X射线光电子能谱(XPS)和共振非弹性X射线散射(RIXS)显示,与SMSI氧化物相互作用后,Pt价态轨道的能量几乎没有变化。由于暴露的Pt位较少,每摩尔Pt对乙烯加氢和丙烷脱氢的催化活性较低,而周转率相似。然而,与Pt / SiO 2相比,SMSI氧化物显着提高了后者反应中丙烯的选择性。在SMSI催化剂中,较高的烯烃选择性被认为是由于暴露的Pt聚集位置较小,从而抑制了烷烃的氢解反应。而暴露的原子仍然具有脱氢活性。
更新日期:2020-09-05
中文翻译:

强金属-载体相互作用(SMSI)对丙烷脱氢用Pt-Ti / SiO2和Pt-Nb / SiO2催化剂的影响
在这项研究中,我们显示了Pt–Nb / SiO 2和Pt–Ti / SiO 2中的强金属-载体相互作用(SMSI)氧化物如何影响丙烷脱氢的电子,几何和催化性能。透射电子显微镜(TEM),CO化学吸附以及每克Pt催化速率的降低证实了Pt纳米颗粒被SMSI氧化物部分覆盖。原位边缘结构(XANES)附近的X射线吸收X射线光电子能谱(XPS)和共振非弹性X射线散射(RIXS)显示,与SMSI氧化物相互作用后,Pt价态轨道的能量几乎没有变化。由于暴露的Pt位较少,每摩尔Pt对乙烯加氢和丙烷脱氢的催化活性较低,而周转率相似。然而,与Pt / SiO 2相比,SMSI氧化物显着提高了后者反应中丙烯的选择性。在SMSI催化剂中,较高的烯烃选择性被认为是由于暴露的Pt聚集位置较小,从而抑制了烷烃的氢解反应。而暴露的原子仍然具有脱氢活性。



























 京公网安备 11010802027423号
京公网安备 11010802027423号