当前位置:
X-MOL 学术
›
Immunology
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Transient tear hyperosmolarity disrupts the neuroimmune homeostasis of the ocular surface and facilitates dry eye onset.
Immunology ( IF 6.4 ) Pub Date : 2020-07-23 , DOI: 10.1111/imm.13243 Mauricio Guzmán 1 , Maximiliano Miglio 1 , Irene Keitelman 1 , Carolina Maiumi Shiromizu 1 , Florencia Sabbione 1 , Federico Fuentes 1 , Analía S Trevani 1 , Mirta N Giordano 1 , Jeremías G Galletti 1
Immunology ( IF 6.4 ) Pub Date : 2020-07-23 , DOI: 10.1111/imm.13243 Mauricio Guzmán 1 , Maximiliano Miglio 1 , Irene Keitelman 1 , Carolina Maiumi Shiromizu 1 , Florencia Sabbione 1 , Federico Fuentes 1 , Analía S Trevani 1 , Mirta N Giordano 1 , Jeremías G Galletti 1
Affiliation
|
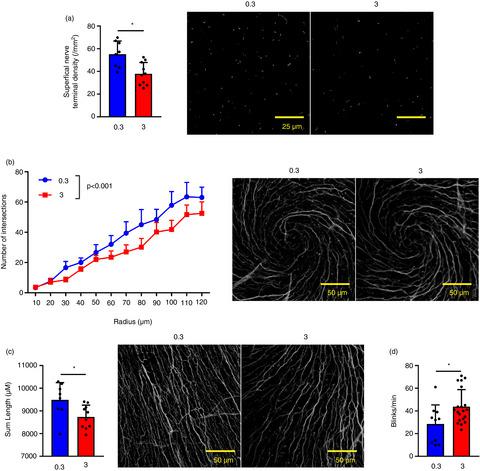
Dry eye disease (DED) is a highly prevalent ocular surface disorder with neuroimmune pathophysiology. Tear hyperosmolarity (THO), a frequent finding in affected patients, is considered a key element in DED pathogenesis, yet existing animal models are based on subjecting the ocular surface to the more complex desiccating stress − decreased tear production and/or increased evaporation − instead of strict hyperosmolar stress. Here we characterized a murine model of THO that does not involve desiccating stress, thus allowing us to dissect the contribution of THO to DED. Our results showed that THO is sufficient to disrupt neuroimmune homeostasis of the ocular surface in mice, and thus reproduce many sub‐clinical DED findings. THO activated nuclear factor‐κB signalling in conjunctival epithelial cells and increased dendritic cell recruitment and maturation, leading to more activated (CD69+) and memory (CD62lo CD44hi) CD4+ T‐cells in the eye‐draining lymph nodes. Ultimately, THO impaired the development of ocular mucosal tolerance to a topical surrogate antigen in a chain of events that included epithelial nuclear factor‐κB signalling and activation of transient receptor potential vanilloid 1 as the probable hypertonicity sensor. Also, THO reduced the density of corneal intraepithelial nerves and terminals, and sensitized the ocular surface to hypertonicity. Finally, the adoptive transfer of T‐cells from THO mice to naïve recipients under mild desiccating stress favoured DED development, showing that THO is enough to trigger an actual pathogenic T‐cell response. Our results altogether demonstrate that THO is a critical initiating factor in DED development.
中文翻译:

短暂的泪液高渗透压会破坏眼表的神经免疫稳态并促进干眼症的发作。
干眼病 (DED) 是一种高度流行的眼表疾病,具有神经免疫病理生理学。泪液高渗 (THO) 是受影响患者的常见发现,被认为是 DED 发病机制的关键因素,但现有的动物模型基于使眼表承受更复杂的干燥压力 - 泪液产生减少和/或蒸发增加 - 相反严格的高渗压力。在这里,我们描述了一个不涉及干燥压力的 THO 小鼠模型,从而使我们能够剖析 THO 对 DED 的贡献。我们的结果表明,THO 足以破坏小鼠眼表的神经免疫稳态,从而重现许多亚临床 DED 发现。+ ) 和记忆 (CD62lo CD44hi) CD4 + T 细胞在眼球引流淋巴结中。最终,THO 在一系列事件中损害了眼粘膜对局部替代抗原的耐受性,包括上皮核因子-κB 信号传导和瞬时受体电位香草素 1 作为可能的高渗传感器的激活。此外,THO 降低了角膜上皮内神经和末梢的密度,并使眼表对高渗敏感。最后,在轻度干燥应激下将 T 细胞从 THO 小鼠过继转移到未接受过的受体有利于 DED 的发展,表明 THO 足以触发实际的致病性 T 细胞反应。我们的结果完全表明 THO 是 DED 开发的关键启动因素。
更新日期:2020-09-18
中文翻译:

短暂的泪液高渗透压会破坏眼表的神经免疫稳态并促进干眼症的发作。
干眼病 (DED) 是一种高度流行的眼表疾病,具有神经免疫病理生理学。泪液高渗 (THO) 是受影响患者的常见发现,被认为是 DED 发病机制的关键因素,但现有的动物模型基于使眼表承受更复杂的干燥压力 - 泪液产生减少和/或蒸发增加 - 相反严格的高渗压力。在这里,我们描述了一个不涉及干燥压力的 THO 小鼠模型,从而使我们能够剖析 THO 对 DED 的贡献。我们的结果表明,THO 足以破坏小鼠眼表的神经免疫稳态,从而重现许多亚临床 DED 发现。+ ) 和记忆 (CD62lo CD44hi) CD4 + T 细胞在眼球引流淋巴结中。最终,THO 在一系列事件中损害了眼粘膜对局部替代抗原的耐受性,包括上皮核因子-κB 信号传导和瞬时受体电位香草素 1 作为可能的高渗传感器的激活。此外,THO 降低了角膜上皮内神经和末梢的密度,并使眼表对高渗敏感。最后,在轻度干燥应激下将 T 细胞从 THO 小鼠过继转移到未接受过的受体有利于 DED 的发展,表明 THO 足以触发实际的致病性 T 细胞反应。我们的结果完全表明 THO 是 DED 开发的关键启动因素。



























 京公网安备 11010802027423号
京公网安备 11010802027423号