Molecular Catalysis ( IF 4.6 ) Pub Date : 2020-07-24 , DOI: 10.1016/j.mcat.2020.111126 Xin Lian , Wenlong Guo , Bai He , Bo Yu , Shuangkou Chen , Dan Qin , Fenglian Chen
|
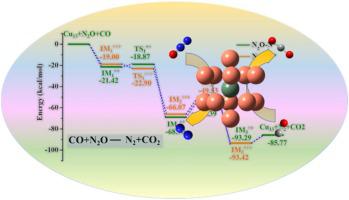
Catalytic conversion of N2O and CO to the nonharmful gases can relieve many environmental problems caused by them. A density functional theory study is performed to investigate the CO oxidation by N2O over the M@Cu12 (M = Cu, Pt, Ru, Pd, Rh) core-shell clusters. The stability of clusters and the adsorption of N2O are enhanced with the doping of noble metals which act as the electron collectors according to the NBO analysis. Two mechanisms (the stepwise adsorption mechanism and the co-adsorption mechanism) with adsorption of N2O on metallic clusters by N end and O end are well-established. On these clusters, the highest activation energy for the N2O decomposition to yield N2 is predicted to be 7.7 kcal/mol. The average barrier for the subsequent CO oxidation is about 14 kcal/mol, which is the rate-limiting step for the overall reaction. Overall, this oxidation process is both thermodynamically and kinetically favorable on Cu-based core-shell clusters. By comparison, the alloy clusters exhibit superior catalytic activities compared to pure Cu cluster for the rate-limiting step.
中文翻译:

关于M @ Cu12(M = Cu,Pt,Ru,Pd,Rh)核-壳簇上N2O氧化CO的机理的见解
将N 2 O和CO催化转化为无害气体可以缓解由它们引起的许多环境问题。进行了密度泛函理论研究,以研究N 2 O在M @ Cu 12(M = Cu,Pt,Ru,Pd,Rh)核-壳簇上的CO氧化。根据NBO分析,通过掺杂用作电子集电器的贵金属,增强了簇的稳定性和N 2 O的吸附。建立了N端和O端在金属团簇上吸附N 2 O的两种机理(逐步吸附机理和共吸附机理)。在这些簇上,N 2 O分解产生N的最高活化能2预计为7.7 kcal / mol。随后的CO氧化的平均势垒为约14kcal / mol,这是整个反应的限速步骤。总体而言,该氧化过程在基于铜的核-壳簇上在热力学和动力学上都是有利的。相比之下,限速步骤中的合金团簇比纯铜团簇具有更好的催化活性。


























 京公网安备 11010802027423号
京公网安备 11010802027423号