Journal of Organometallic Chemistry ( IF 2.3 ) Pub Date : 2020-07-24 , DOI: 10.1016/j.jorganchem.2020.121456 Laura M. Thierer , Sarah E. Jenny , Vaidehi Shastri , Marianne R. Donley , Lindsey M. Round , Nicholas A. Piro , W. Scott Kassel , Catherine L. Brown , Timothy J. Dudley , Deanna L. Zubris
|
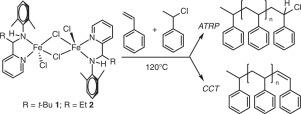
The amino-pyridine ligand scaffold has achieved widespread use for base metal catalysis. Atom Transfer Radical Polymerization (ATRP) is one realm where base metals have achieved success in catalysis, in particular copper and iron. Herein, the synthesis and characterization of two amino-pyridine iron(II) complexes is described where the amino carbon substitution is the point of differentiation. We hypothesized that a sterically hindered, electron rich t-butyl substituent in this position might improve the propensity of said complex to achieve ATRP since inductive electron donation from the t-butyl group may improve catalyst activity and shift the ATRP equilibrium towards the active polymer species and corresponding Fe(III) complex. Dimeric 1 and 2 ([2-[(2,6-Me2-C6H3)NHCH(R)]C5H4N]FeCl2)2 (R = t-butyl or ethyl, respectively) were identified by single crystal X-ray diffraction. Both complexes favor a high-spin iron(II) state, as evidenced by Evans NMR magnetic susceptibility measurements and suggested by gas-phase computations at the M06-L level of theory. Complexes 1 and 2 catalyze styrene polymerization at elevated temperatures (120 °C) and polymerization data suggests that ATRP operates and catalytic chain transfer (CCT) competes at extended reaction times. Complex 1 with its t-butyl substituted amino carbon displays a slightly higher ATRP activity as compared to 2 [kobs(1) = 0.31 h−1; kobs(2) = 0.10 h−1], suggesting the importance of ligand optimization for future iron ATRP catalyst development.
中文翻译:

氨基吡啶铁(II)配合物:原子转移自由基聚合和催化链转移的表征和催化应用
氨基吡啶配体支架已广泛用于贱金属催化。原子转移自由基聚合(ATRP)是贱金属(尤其是铜和铁)在催化领域取得成功的领域。在本文中,描述了两种氨基吡啶铁(II)配合物的合成和表征,其中氨基碳取代是区别点。我们假设在该位置存在空间位阻的,富电子的叔丁基取代基可能会改善所述络合物实现ATRP的倾向,因为从叔丁基进行的感应电子给体可能会改善催化剂活性并使ATRP平衡向活性聚合物转移以及相应的Fe(III)配合物 二聚体1和2([2-[(2,6-Me 2 -C 6 H 3)NHCH(R)] C 5 H 4 N] FeCl 2)2(R分别为 叔丁基或乙基)通过单晶X鉴定射线衍射。两种络合物均倾向于高自旋铁(II)态,这通过Evans NMR磁化率测量得到了证明,并且在M06-L理论水平上通过气相计算得到了证明。配合物1和2在升高的温度(120°C)下催化苯乙烯聚合,聚合数据表明ATRP起作用,并且催化链转移(CCT)在延长的反应时间中竞争。复数1及其t丁基取代的氨基碳与2 [ k obs(1)= 0.31 h -1 ; k obs(2)= 0.10 h -1 ],表明配体优化对未来铁ATRP催化剂开发的重要性。


























 京公网安备 11010802027423号
京公网安备 11010802027423号