Biochimie ( IF 3.9 ) Pub Date : 2020-07-24 , DOI: 10.1016/j.biochi.2020.07.007 Pushpa Mishra 1 , Crismita Dmello 2 , Disha Sengupta 2 , Suraj Chandrabhan Singh 3 , Nikita Kirkise 2 , Ramakrishna V Hosur 4 , Shobhona Sharma 2
|
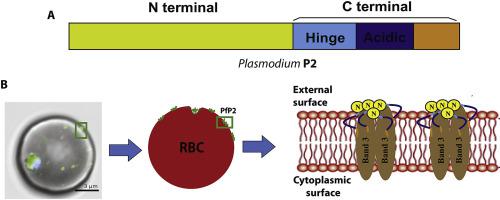
The ribosomal protein P2 of Plasmodium falciparum, (PfP2), performs certain unique extra-ribosomal functions. During the few hours of cell-division, PfP2 protein moves to the external surface of the infected erythrocytes (IE) as an SDS-resistant oligomer, and at that stage treatment with specific anti- PfP2 antibodies results in an arrest of the parasite cell-division. Amongst the oligomeric forms of PfP2, mainly the homo-tetramer is peripherally anchored on the external surface of the IE. To study the anchoring of PfP2 tetramer on IE-surface, we have explored the binding properties of PfP2 protein. Using NMR and erythrocyte pull-down studies, here we report that the homo-tetrameric PfP2 protein interacted specifically with erythrocytes and not leukocytes. The hydrophobic N-terminal 72 amino acid region is the major interacting domain. The binding of P2 to RBCs was neuraminidase resistant, but trypsin sensitive. The RBC binding was exclusive to the Plasmodium PfP2 protein as even the homologous protein of the closely related Apicomplexan parasite Toxoplasma gondii TgP2 protein did not interact with erythrocytes. Pull down assays, immunoprecipitation and mass spectrometry data showed that erythrocytic Band 3 protein is a possible interactor of Plasmodium PfP2 protein on the erythrocyte surface.
中文翻译:

疟原虫核糖体蛋白P2与红细胞结合的分子研究。
恶性疟原虫的核糖体蛋白P2(PfP2),执行某些独特的核糖体外功能。在细胞分裂的数小时内,PfP2蛋白以抗SDS的寡聚体的形式移动到被感染的红细胞(IE)的外表面,在那个阶段,用特定的抗PfP2抗体进行治疗会导致寄生虫细胞的停滞。师。在PfP2的寡聚形式中,同型四聚体主要锚定在IE的外表面。为了研究PfP2四聚体在IE表面上的锚定,我们探索了PfP2蛋白的结合特性。使用核磁共振和红细胞下拉研究,在这里我们报道同四聚体PfP2蛋白与红细胞而不是白细胞特异性相互作用。疏水的N末端72个氨基酸区域是主要的相互作用域。P2与RBC的结合具有神经氨酸酶抗性,但对胰蛋白酶敏感。RBC绑定仅适用于疟原虫PfP2蛋白甚至是紧密相关的Apicomplexan寄生虫弓形虫TgP2蛋白的同源蛋白,也不会与红细胞相互作用。下拉分析,免疫沉淀和质谱数据显示,红细胞带3蛋白可能是红细胞表面上疟原虫PfP2蛋白的可能相互作用物。


























 京公网安备 11010802027423号
京公网安备 11010802027423号