当前位置:
X-MOL 学术
›
J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Synthesis, Characterization, and Application of a Highly Hydrophilic Triarylmethyl Radical for Biomedical EPR.
The Journal of Organic Chemistry ( IF 3.6 ) Pub Date : 2020-07-22 , DOI: 10.1021/acs.joc.0c00557 Urikhan Sanzhaeva 1, 2 , Martin Poncelet 1, 3 , Oxana Tseytlin 1, 2 , Mark Tseytlin 1, 2 , Marieta Gencheva 1, 4 , Timothy D Eubank 1, 4 , Valery V Khramtsov 1, 2 , Benoit Driesschaert 1, 3
The Journal of Organic Chemistry ( IF 3.6 ) Pub Date : 2020-07-22 , DOI: 10.1021/acs.joc.0c00557 Urikhan Sanzhaeva 1, 2 , Martin Poncelet 1, 3 , Oxana Tseytlin 1, 2 , Mark Tseytlin 1, 2 , Marieta Gencheva 1, 4 , Timothy D Eubank 1, 4 , Valery V Khramtsov 1, 2 , Benoit Driesschaert 1, 3
Affiliation
|
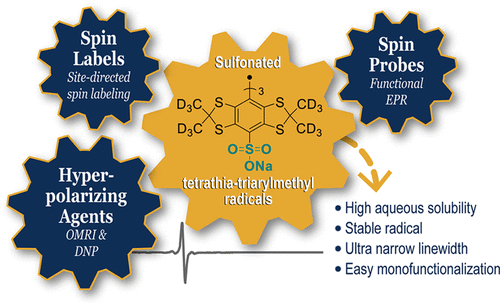
Stable tetrathiatriarylmethyl radicals have significantly contributed to the recent progress in biomedical electron paramagnetic resonance (EPR) due to their unmatched stability in biological media and long relaxation times. However, the lipophilic core of the most commonly used structure (Finland trityl) is responsible for its interaction with plasma biomacromolecules, such as albumin, and self-aggregation at high concentrations and/or low pH. While Finland trityl is generally considered inert toward many reactive radical species, we report that sulfite anion radical efficiently substitutes the three carboxyl moieties of Finland trityl with a high rate constant of 3.53 × 108 M–1 s–1, leading to a trisulfonated Finland trityl radical. This newly synthesized highly hydrophilic trityl radical shows an ultranarrow linewidth (ΔBpp = 24 mG), a lower affinity for albumin than Finland trityl, and a high aqueous solubility even at acidic pH. Therefore, this new tetrathiatriarylmethyl radical can be considered as a superior spin probe in comparison to the widely used Finland trityl. One of its potential applications was demonstrated by in vivo mapping oxygen in a mouse model of breast cancer. Moreover, we showed that one of the three sulfo groups can be easily substituted with S-, N-, and P-nucleophiles, opening access to various monofunctionalized sulfonated trityl radicals.
中文翻译:

用于生物医学 EPR 的高亲水性三芳基甲基自由基的合成、表征和应用。
由于其在生物介质中无与伦比的稳定性和较长的弛豫时间,稳定的四硫三芳基甲基自由基对生物医学电子顺磁共振 (EPR) 的最新进展做出了重大贡献。然而,最常用结构(芬兰三苯甲基)的亲脂性核心负责其与血浆生物大分子(如白蛋白)的相互作用,以及在高浓度和/或低 pH 值下的自聚集。虽然芬兰三苯甲基通常被认为对许多活性自由基物种呈惰性,但我们报告说,亚硫酸盐阴离子自由基有效地取代了芬兰三苯甲基的三个羧基部分,具有 3.53 × 10 8 M –1 s –1的高速率常数,导致三磺化芬兰三苯甲基自由基。这种新合成的高亲水性三苯甲基自由基显示出超窄线宽 (Δ B pp = 24 mG),对白蛋白的亲和力低于芬兰三苯甲基,并且即使在酸性 pH 下也具有高水溶性。因此,与广泛使用的芬兰三苯甲基相比,这种新的四硫三芳基甲基自由基可以被认为是一种优越的自旋探针。它的潜在应用之一是通过在乳腺癌小鼠模型中体内测绘氧气来证明的。此外,我们表明三个磺基中的一个可以很容易地被 S-、N-和 P-亲核试剂取代,从而打开了获得各种单官能化磺化三苯甲基自由基的途径。
更新日期:2020-08-21
中文翻译:

用于生物医学 EPR 的高亲水性三芳基甲基自由基的合成、表征和应用。
由于其在生物介质中无与伦比的稳定性和较长的弛豫时间,稳定的四硫三芳基甲基自由基对生物医学电子顺磁共振 (EPR) 的最新进展做出了重大贡献。然而,最常用结构(芬兰三苯甲基)的亲脂性核心负责其与血浆生物大分子(如白蛋白)的相互作用,以及在高浓度和/或低 pH 值下的自聚集。虽然芬兰三苯甲基通常被认为对许多活性自由基物种呈惰性,但我们报告说,亚硫酸盐阴离子自由基有效地取代了芬兰三苯甲基的三个羧基部分,具有 3.53 × 10 8 M –1 s –1的高速率常数,导致三磺化芬兰三苯甲基自由基。这种新合成的高亲水性三苯甲基自由基显示出超窄线宽 (Δ B pp = 24 mG),对白蛋白的亲和力低于芬兰三苯甲基,并且即使在酸性 pH 下也具有高水溶性。因此,与广泛使用的芬兰三苯甲基相比,这种新的四硫三芳基甲基自由基可以被认为是一种优越的自旋探针。它的潜在应用之一是通过在乳腺癌小鼠模型中体内测绘氧气来证明的。此外,我们表明三个磺基中的一个可以很容易地被 S-、N-和 P-亲核试剂取代,从而打开了获得各种单官能化磺化三苯甲基自由基的途径。


























 京公网安备 11010802027423号
京公网安备 11010802027423号