当前位置:
X-MOL 学术
›
Chin. J. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Manganese‐Catalyzed Hydrocarbofunctionalization of Internal Alkenes†
Chinese Journal of Chemistry ( IF 5.4 ) Pub Date : 2020-07-22 , DOI: 10.1002/cjoc.202000376 Dongping Wang 1 , Yijie He 1 , Haotian Dai 1 , Congcong Huang 2 , Xiang‐Ai Yuan 2 , Jin Xie 1
Chinese Journal of Chemistry ( IF 5.4 ) Pub Date : 2020-07-22 , DOI: 10.1002/cjoc.202000376 Dongping Wang 1 , Yijie He 1 , Haotian Dai 1 , Congcong Huang 2 , Xiang‐Ai Yuan 2 , Jin Xie 1
Affiliation
|
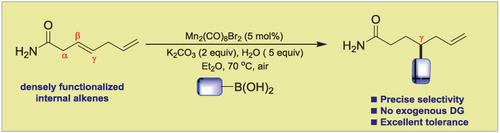
A highly regio‐ and chemo‐selective dimeric Mn(I)‐catalyzed hydroarylation and hydroalkenylation of unbiased internal alkenes with commercially abundant organoboron compounds is reported. A wide range of densely functionalized aliphatic alkenyl amides can successfully undergo site‐exclusive hydrocarbofunctionalization in air atmosphere without any exogenous directing auxiliary, affording an enhanced lead library of structurally diverse γ‐functionalized carboxylic acid derivatives in moderate to good yields. The precise chemoselectivity of the reaction among multiple alkene units even in the presence of reactive terminal alkenes highlights the unique catalytic features of manganese catalyst, and the excellent functional group compatibility of primary amides further complements other transition metals.
中文翻译:

内烯烃的锰催化烃官能化†
据报道,具有高区域和化学选择性的二聚体Mn(I)催化无偏内烯烃与商业上丰富的有机硼化合物的加氢芳基化和加氢烯基化反应。各种各样的稠密官能化的脂肪族烯基酰胺可以在空气中成功进行位点排他的烃官能化反应,而无需任何外源性的导向助剂,从而以中等至良好的产率提供了结构多样的γ官能化羧酸衍生物的增强的铅库。即使在反应性末端烯烃的存在下,多个烯烃单元之间反应的精确化学选择性也突出了锰催化剂的独特催化特性,伯酰胺出色的官能团相容性进一步补充了其他过渡金属。
更新日期:2020-07-22
中文翻译:

内烯烃的锰催化烃官能化†
据报道,具有高区域和化学选择性的二聚体Mn(I)催化无偏内烯烃与商业上丰富的有机硼化合物的加氢芳基化和加氢烯基化反应。各种各样的稠密官能化的脂肪族烯基酰胺可以在空气中成功进行位点排他的烃官能化反应,而无需任何外源性的导向助剂,从而以中等至良好的产率提供了结构多样的γ官能化羧酸衍生物的增强的铅库。即使在反应性末端烯烃的存在下,多个烯烃单元之间反应的精确化学选择性也突出了锰催化剂的独特催化特性,伯酰胺出色的官能团相容性进一步补充了其他过渡金属。


























 京公网安备 11010802027423号
京公网安备 11010802027423号