当前位置:
X-MOL 学术
›
Stem Cells Transl. Med.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
CRISPR/Cas9-mediated introduction of the sodium/iodide symporter gene enables noninvasive in vivo tracking of induced pluripotent stem cell-derived cardiomyocytes.
STEM CELLS Translational Medicine ( IF 6 ) Pub Date : 2020-07-23 , DOI: 10.1002/sctm.20-0019 John W Ostrominski 1 , Ravi Chandra Yada 1 , Noriko Sato 2 , Michael Klein 3 , Ksenia Blinova 4 , Dakshesh Patel 4 , Racquel Valadez 5 , Maryknoll Palisoc 5 , Stefania Pittaluga 5 , Kah-Whye Peng 6 , Hong San 7 , Yongshun Lin 8 , Falguni Basuli 9 , Xiang Zhang 9 , Rolf E Swenson 9 , Mark Haigney 3 , Peter L Choyke 2 , Jizhong Zou 8 , Manfred Boehm 10 , So Gun Hong 1 , Cynthia E Dunbar 1
STEM CELLS Translational Medicine ( IF 6 ) Pub Date : 2020-07-23 , DOI: 10.1002/sctm.20-0019 John W Ostrominski 1 , Ravi Chandra Yada 1 , Noriko Sato 2 , Michael Klein 3 , Ksenia Blinova 4 , Dakshesh Patel 4 , Racquel Valadez 5 , Maryknoll Palisoc 5 , Stefania Pittaluga 5 , Kah-Whye Peng 6 , Hong San 7 , Yongshun Lin 8 , Falguni Basuli 9 , Xiang Zhang 9 , Rolf E Swenson 9 , Mark Haigney 3 , Peter L Choyke 2 , Jizhong Zou 8 , Manfred Boehm 10 , So Gun Hong 1 , Cynthia E Dunbar 1
Affiliation
|
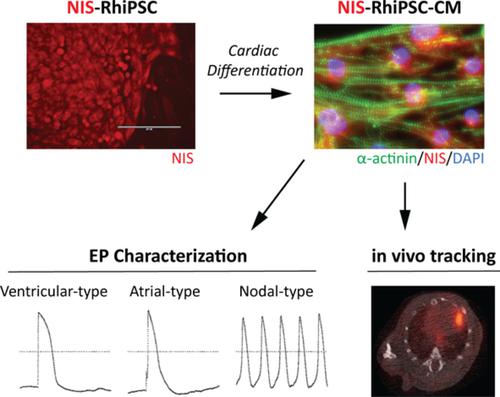
Techniques that enable longitudinal tracking of cell fate after myocardial delivery are imperative for optimizing the efficacy of cell‐based cardiac therapies. However, these approaches have been underutilized in preclinical models and clinical trials, and there is considerable demand for site‐specific strategies achieving long‐term expression of reporter genes compatible with safe noninvasive imaging. In this study, the rhesus sodium/iodide symporter (NIS) gene was incorporated into rhesus macaque induced pluripotent stem cells (RhiPSCs) via CRISPR/Cas9. Cardiomyocytes derived from NIS‐RhiPSCs (NIS‐RhiPSC‐CMs) exhibited overall similar morphological and electrophysiological characteristics compared to parental control RhiPSC‐CMs at baseline and with exposure to physiological levels of sodium iodide. Mice were injected intramyocardially with 2 million NIS‐RhiPSC‐CMs immediately following myocardial infarction, and serial positron emission tomography/computed tomography was performed with 18F‐tetrafluoroborate to monitor transplanted cells in vivo. NIS‐RhiPSC‐CMs could be detected until study conclusion at 8 to 10 weeks postinjection. This NIS‐based molecular imaging platform, with optimal safety and sensitivity characteristics, is primed for translation into large‐animal preclinical models and clinical trials.
中文翻译:

CRISPR/Cas9 介导的钠/碘化物同向转运体基因的引入能够在体内无创追踪诱导多能干细胞衍生的心肌细胞。
能够在心肌递送后纵向追踪细胞命运的技术对于优化基于细胞的心脏疗法的功效至关重要。然而,这些方法在临床前模型和临床试验中未得到充分利用,并且对实现与安全无创成像兼容的报告基因的长期表达的位点特异性策略有相当大的需求。在这项研究中,恒河猴钠/碘同向转运体 (NIS) 基因通过 CRISPR/Cas9 整合到恒河猴诱导的多能干细胞 (RhiPSCs) 中。与亲本对照 RhiPSC-CM 相比,NIS-RhiPSC 衍生的心肌细胞 (NIS-RhiPSC-CM) 在基线和生理水平的碘化钠暴露下表现出总体相似的形态学和电生理特征。18 F-四氟硼酸盐用于监测体内移植细胞。在注射后 8 至 10 周的研究结束之前,可以检测到 NIS-RhiPSC-CM。这种基于 NIS 的分子成像平台具有最佳的安全性和灵敏度特性,已准备好转化为大型动物临床前模型和临床试验。
更新日期:2020-09-26
中文翻译:

CRISPR/Cas9 介导的钠/碘化物同向转运体基因的引入能够在体内无创追踪诱导多能干细胞衍生的心肌细胞。
能够在心肌递送后纵向追踪细胞命运的技术对于优化基于细胞的心脏疗法的功效至关重要。然而,这些方法在临床前模型和临床试验中未得到充分利用,并且对实现与安全无创成像兼容的报告基因的长期表达的位点特异性策略有相当大的需求。在这项研究中,恒河猴钠/碘同向转运体 (NIS) 基因通过 CRISPR/Cas9 整合到恒河猴诱导的多能干细胞 (RhiPSCs) 中。与亲本对照 RhiPSC-CM 相比,NIS-RhiPSC 衍生的心肌细胞 (NIS-RhiPSC-CM) 在基线和生理水平的碘化钠暴露下表现出总体相似的形态学和电生理特征。18 F-四氟硼酸盐用于监测体内移植细胞。在注射后 8 至 10 周的研究结束之前,可以检测到 NIS-RhiPSC-CM。这种基于 NIS 的分子成像平台具有最佳的安全性和灵敏度特性,已准备好转化为大型动物临床前模型和临床试验。


























 京公网安备 11010802027423号
京公网安备 11010802027423号