Nanomedicine: Nanotechnology, Biology and Medicine ( IF 5.4 ) Pub Date : 2020-07-23 , DOI: 10.1016/j.nano.2020.102274 Moran Levi 1 , Mark Epshtein 1 , Tatsiana Castor 2 , Meinrad Gawaz 2 , Netanel Korin 1
|
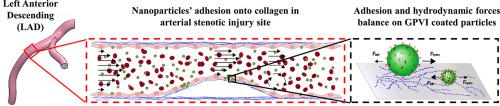
Thrombus formation at athero-thrombotic sites is initiated by the exposure of collagen followed by platelet adhesion mediated by the platelet-specific collagen receptor glycoprotein VI (GPVI). Here, dimeric GPVI was used as a targeting motif to functionalize polymeric nanoparticle-based drug carriers and to show that with proper design, such GPVI-coated nanoparticles (GPNs) can efficiently and specifically target arterial injury sites while withstanding physiological flow. In a microfluidic model, under physiological shear levels (1-40 dyne/cm2), 200 nm and 2 μm GPNs exhibited a >60 and >10-fold increase in binding to collagen compared to control particles, respectively. In vitro experiments in an arterial stenosis injury model, subjected to physiological pulsatile flow, showed shear-enhanced adhesion of 200 nm GPNs at the stenosis region which was confirmed in vivo in a mice ligation carotid injury model using intravital microscopy. Altogether, our results illustrate how engineering tools can be harnessed to design nano-carriers that efficiently target cardiovascular disease sites.
中文翻译:

糖蛋白VI(GPVI)功能化的纳米粒子靶向生理流下的动脉损伤部位。
动脉粥样硬化血栓形成部位的血栓形成是由胶原蛋白的暴露引起的,然后由血小板特异性胶原蛋白受体糖蛋白VI(GPVI)介导的血小板粘附引起。在这里,二聚体GPVI被用作靶向基序,以功能化基于聚合物纳米颗粒的药物载体,并显示出通过适当的设计,这种GPVI包覆的纳米颗粒(GPN)可以有效且特异性地靶向动脉损伤部位,同时承受生理流。在微流模型中,在生理剪切水平下(1-40达因/ cm 2),与对照颗粒相比,200 nm和2μm的GPNs与胶原蛋白的结合分别表现出> 60和> 10倍的增加。在经受生理脉动流的动脉狭窄损伤模型中的体外实验显示,狭窄区域200 nm GPNs的剪切增强粘附力,这在活体显微镜下的小鼠结扎颈动脉损伤模型中得到了证实。总而言之,我们的结果说明了如何利用工程工具来设计可有效靶向心血管疾病部位的纳米载体。



























 京公网安备 11010802027423号
京公网安备 11010802027423号