Chem ( IF 23.5 ) Pub Date : 2020-07-23 , DOI: 10.1016/j.chempr.2020.06.031 Zhaohong Liu , Shanshan Cao , Weijie Yu , Jiayi Wu , Fanhua Yi , Edward A. Anderson , Xihe Bi
|
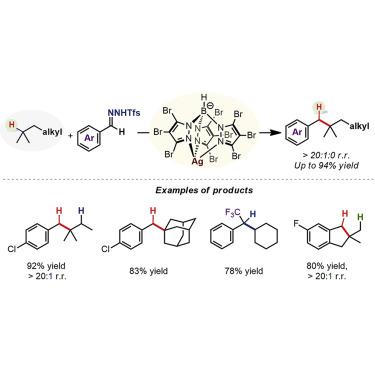
Alkanes are an abundant and valuable resource for transformation into value-added fine chemicals. However, selective functionalization of specific C(sp3)–H bonds in alkanes for alkyl-alkyl bond formation is a significant challenge because of their intrinsic inertness and the small differences in reactivity among various C(sp3)–H bonds. Here, we report a silver-catalyzed site-selective C(sp3)–H benzylation of simple alkanes using N-triftosylhydrazones as a convenient carbene precursor, which enables the synthesis of high value-added alkylated aromatics. A one-pot two-step protocol starting from aldehydes was also realized, thereby constituting a formal reductive alkylation of aryl aldehydes by alkanes. Experimental investigations and DFT calculations reveal that the role of the [TpBr3Ag]2 catalyst is 3-fold: it modulates the carbene reactivity, inhibits carbene dimerization, and avoids over insertion of the product. All three aspects are crucial for the success of this first site-selective intermolecular insertion of donor carbenes into C(sp3)–H bonds of simple alkanes.
中文翻译:

烷烃与N -Triftosylhydrazones的站点选择性CH苯甲酰化导致烷基芳烃。
烷烃是转化为高附加值精细化学品的宝贵资源。但是,由于烷烃固有的惰性以及各种C(sp 3)-H键之间的反应性差异小,因此烷烃中特定的C(sp 3)-H键选择性官能化以形成烷基-烷基键是一项重大挑战。在这里,我们报告使用N的简单烷烃的银催化位点选择性C(sp 3)–H苄基化-triftosylhydrazones作为方便的卡宾前体,可以合成高附加值的烷基化芳烃。还实现了从醛开始的一锅两步方案,从而构成了烷烃对芳基醛的正式还原烷基化。实验研究和DFT计算表明[Tp Br3 Ag] 2催化剂的作用是3倍:它调节卡宾反应性,抑制卡宾二聚作用,并避免产物过度插入。所有这三个方面对于将供体卡宾首次分子间分子插入简单烷烃的C(sp 3)-H键的成功至关重要。



























 京公网安备 11010802027423号
京公网安备 11010802027423号