Biochimica et Biophysica Acta (BBA) - Molecular Cell Research ( IF 5.1 ) Pub Date : 2020-07-23 , DOI: 10.1016/j.bbamcr.2020.118801 Fiyaz Mohammed 1 , Catharine Trieber 2 , Michael Overduin 2 , Martyn Chidgey 3
|
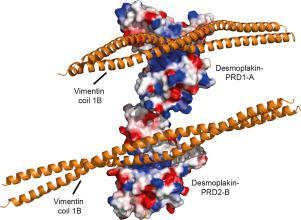
The plakin family of cytolinkers interacts with intermediate filaments (IFs) through plakin repeat domain (PRD) and linker modules. Recent structure/function studies have established the molecular basis of envoplakin-PRD and periplakin-linker interactions with vimentin. Both plakin modules share a broad basic groove which recognizes acidic rod elements on IFs, a mechanism that is applicable to other plakin family members. This review postulates a universal IF engagement mechanism that illuminates the specific effects of pathogenic mutations associated with diseases including arrhythmogenic right ventricular cardiomyopathy, and reveals how diverse plakin proteins offer tailored IF tethering to ensure stable, dynamic and regulated cellular structures.
中文翻译:

普通蛋白识别中间丝的分子机制。
plakin细胞连接子家族通过plakin重复域(PRD)和连接子模块与中间丝(IF)相互作用。最近的结构/功能研究已经建立了envoplakin-PRD和periplakin-linker与波形蛋白相互作用的分子基础。两个plakin模块均具有宽阔的基本凹槽,可识别IF上的酸性杆元件,该机制适用于其他plakin家族成员。这篇综述提出了一种通用的IF参与机制,该机制阐明了与包括心律失常性右心室心肌病在内的疾病相关的致病突变的特定作用,并揭示了各种不同的普通蛋白如何提供定制的IF系链以确保稳定,动态和受调节的细胞结构。


























 京公网安备 11010802027423号
京公网安备 11010802027423号