Tetrahedron Letters ( IF 1.8 ) Pub Date : 2020-07-22 , DOI: 10.1016/j.tetlet.2020.152277 Alexey V. Zakharov , Evgenia A. Mitina , Valerii Z. Shirinian
|
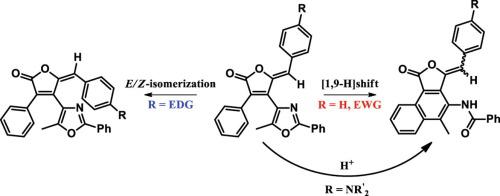
In recent years, the synthetic potential of the photorearrangement of diarylethenes leading to naphthalene and phenanthrene derivatives via a cascade process of photocyclization/[1,9]-H shift has been demonstrated. In this work, the influence of various substituents on the efficiency of the photorearrangement of diarylethenes (DAEs) of the furanone series containing an additional π-system has been explored. It was found that electron-withdrawing substituents contribute only to the photocyclization process, while electron-donating groups (methoxy or dialkylamino groups) lead to E/Z-isomerization. However, in the case of dialkylamino substituted diarylethenes, the addition of a strong Brønsted acid promotes the photorearrangement, but prevents E/Z isomerization.
中文翻译:

呋喃酮二芳基乙烯的合成及photorearrangement有附加π -系统
近年来,已经证明了通过光环化/ [1,9] -H转换的级联过程,导致萘和菲衍生物的二芳烃的光重排的合成潜力。在这项工作中,已经探索了各种取代基对含有额外的π-系统的呋喃酮系列的二芳烃(DAE)的光重排效率的影响。发现吸电子取代基仅有助于光环化过程,而给电子基团(甲氧基或二烷基氨基)导致E / Z-异构化。但是,对于二烷基氨基取代的二芳烃,添加强布朗斯台德酸会促进光重排,但会阻止E / Z 异构化。


























 京公网安备 11010802027423号
京公网安备 11010802027423号