当前位置:
X-MOL 学术
›
Chin. J. Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Morphology evolution of acetic acid-modulated MIL-53(Fe) for efficient selective oxidation of H2S
Chinese Journal of Catalysis ( IF 16.5 ) Pub Date : 2021-02-01 , DOI: 10.1016/s1872-2067(20)63625-7 Xiaoxiao Zheng , Sihui Qi , Yanning Cao , Lijuan Shen , Chaktong Au , Lilong Jiang
Chinese Journal of Catalysis ( IF 16.5 ) Pub Date : 2021-02-01 , DOI: 10.1016/s1872-2067(20)63625-7 Xiaoxiao Zheng , Sihui Qi , Yanning Cao , Lijuan Shen , Chaktong Au , Lilong Jiang
|
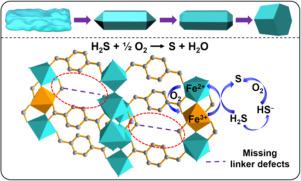
Abstract MIL-53(Fe) was synthesized using a “modulator approach” that utilizes acetic acid (HAc) as an additive to control the size and morphology of the resulting crystals. We demonstrate that after activation under vaccum at 100 °C, the MIL-53(Fe) functions well for H2S selective oxidation. The introduction of acetic acid in the presence of benzene-1,4-dicarboxylic acid (H2BDC) would result in a series of MIL-53(Fe) nanocrystals (denoted as MIL-53(Fe)-xH, x stands for the volume of added HAc with morphology evoluting from irregular particles to short hexagonal columns. The vacuum treatment facilitates the removal of acetate groups, thus generating Fe3+ Lewis acid sites. Consequently, the resulted MIL-53(Fe)-xH exhibits good catalytic activity (98% H2S conversion and 92% sulfur selectivity) at moderate reaction temperatures (100–190 °C). The MIL-53(Fe)-5H is superior to the traditional iron-based catalysts, showing stable performance in a test period of 55 h.
中文翻译:

乙酸调制的 MIL-53(Fe) 的形态演变以有效选择性氧化 H2S
摘要 MIL-53(Fe) 是使用“调节剂方法”合成的,该方法利用乙酸 (HAc) 作为添加剂来控制所得晶体的尺寸和形态。我们证明,在 100 °C 真空下活化后,MIL-53(Fe) 可以很好地用于 H2S 选择性氧化。在苯-1,4-二羧酸 (H2BDC) 存在下引入乙酸将产生一系列 MIL-53(Fe) 纳米晶体(表示为 MIL-53(Fe)-xH,x 代表体积添加的HAc形态从不规则颗粒演变为短六角柱。真空处理有利于去除乙酸基团,从而产生Fe3+路易斯酸位点。因此,所得的MIL-53(Fe)-xH表现出良好的催化活性(98%) H2S 转化率和 92% 的硫选择性)在中等反应温度(100–190 °C)。
更新日期:2021-02-01
中文翻译:

乙酸调制的 MIL-53(Fe) 的形态演变以有效选择性氧化 H2S
摘要 MIL-53(Fe) 是使用“调节剂方法”合成的,该方法利用乙酸 (HAc) 作为添加剂来控制所得晶体的尺寸和形态。我们证明,在 100 °C 真空下活化后,MIL-53(Fe) 可以很好地用于 H2S 选择性氧化。在苯-1,4-二羧酸 (H2BDC) 存在下引入乙酸将产生一系列 MIL-53(Fe) 纳米晶体(表示为 MIL-53(Fe)-xH,x 代表体积添加的HAc形态从不规则颗粒演变为短六角柱。真空处理有利于去除乙酸基团,从而产生Fe3+路易斯酸位点。因此,所得的MIL-53(Fe)-xH表现出良好的催化活性(98%) H2S 转化率和 92% 的硫选择性)在中等反应温度(100–190 °C)。



























 京公网安备 11010802027423号
京公网安备 11010802027423号