当前位置:
X-MOL 学术
›
React. Chem. Eng.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Kinetic modelling of hydrogen transfer deoxygenation of a prototypical fatty acid over a bimetallic Pd60Cu40 catalyst: an investigation of the surface reaction mechanism and rate limiting step
Reaction Chemistry & Engineering ( IF 3.9 ) Pub Date : 2020-07-21 , DOI: 10.1039/d0re00214c Kin Wai Cheah 1, 2, 3, 4, 5 , Suzana Yusup 1, 2, 3, 4, 5 , Martin J. Taylor 6, 7, 8, 9 , Bing Shen How 10, 11, 12, 13, 14 , Amin Osatiashtiani 9, 15, 16, 17 , Daniel J. Nowakowski 9, 15, 16, 17 , Anthony V. Bridgwater 9, 15, 16, 17 , Vasiliki Skoulou 6, 7, 8, 9 , Georgios Kyriakou 4, 18, 19, 20 , Yoshitmitsu Uemura 1, 2, 3, 4, 5
Reaction Chemistry & Engineering ( IF 3.9 ) Pub Date : 2020-07-21 , DOI: 10.1039/d0re00214c Kin Wai Cheah 1, 2, 3, 4, 5 , Suzana Yusup 1, 2, 3, 4, 5 , Martin J. Taylor 6, 7, 8, 9 , Bing Shen How 10, 11, 12, 13, 14 , Amin Osatiashtiani 9, 15, 16, 17 , Daniel J. Nowakowski 9, 15, 16, 17 , Anthony V. Bridgwater 9, 15, 16, 17 , Vasiliki Skoulou 6, 7, 8, 9 , Georgios Kyriakou 4, 18, 19, 20 , Yoshitmitsu Uemura 1, 2, 3, 4, 5
Affiliation
|
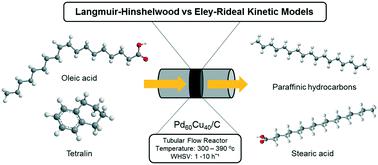
Herein, for the first time, we demonstrate a novel continuous flow process involving the application of tetralin as a hydrogen donor solvent for the catalytic conversion of oleic acid to diesel-like hydrocarbons, using an efficient and stable carbon-supported bimetallic PdCu catalyst. Using Pd60Cu40/C, where 60 : 40 is the molar ratio of each metal, at optimum reaction conditions (360 °C and WHSV = 1 h−1), 90.5% oleic acid conversion and 80.5% selectivity to C17 and C18 paraffinic hydrocarbons were achieved. Furthermore, a comprehensive mechanistic based kinetic modelling – considering power rate law, L–H and E–R models was conducted. Kinetic expressions derived from the three kinetic models were investigated in rate data fitting through nonlinear regression using a Levenberg–Marquardt algorithm. Based on the statistical discrimination criteria, the experimental data of the dehydrogenation reaction of tetralin were best fitted by an L–H rate equation assuming the surface reaction as the rate controlling step. In contrast, the kinetic data of the oleic acid deoxygenation reaction were well correlated with an L–H rate equation assuming single site adsorption of oleic acid with dissociative H2 adsorption. It was found that the rate limiting step of the overall reaction was the hydrogenation of oleic acid with an activation energy of 75.0 ± 5.1 kJ mol−1 whereas the dehydrogenation of tetralin had a lower activation energy of 66.4 ± 2.7 kJ mol−1.
中文翻译:

双金属Pd60Cu40催化剂上原型脂肪酸氢转移脱氧的动力学模型:表面反应机理和限速步骤的研究
在此,我们首次展示了一种新颖的连续流工艺,该工艺涉及使用四氢萘作为氢供体溶剂,使用高效且稳定的碳载双金属PdCu催化剂将油酸催化转化为类柴油。使用Pd 60 Cu 40 / C,其中60:40是每种金属的摩尔比,在最佳反应条件(360°C和WHSV = 1 h -1)下,油酸转化率为90.5%,对C 17和C的选择性为80.5%。C 18实现了石蜡烃。此外,还进行了基于机理的综合动力学建模-考虑了电费率定律,L–H和E–R模型。使用Levenberg-Marquardt算法通过非线性回归在速率数据拟合中研究了从这三种动力学模型得出的动力学表达式。根据统计判别标准,以表面反应为速率控制步骤,通过L–H速率方程可以最佳拟合四氢化萘脱氢反应的实验数据。相反,假设油酸与解离性H 2单点吸附,油酸脱氧反应的动力学数据与L–H速率方程密切相关。吸附。发现整个反应的限速步骤是油酸的氢化,其活化能为75.0±5.1kJ mol -1,而四氢化萘的脱氢具有较低的活化能为66.4±2.7kJ mol -1。
更新日期:2020-08-25
中文翻译:

双金属Pd60Cu40催化剂上原型脂肪酸氢转移脱氧的动力学模型:表面反应机理和限速步骤的研究
在此,我们首次展示了一种新颖的连续流工艺,该工艺涉及使用四氢萘作为氢供体溶剂,使用高效且稳定的碳载双金属PdCu催化剂将油酸催化转化为类柴油。使用Pd 60 Cu 40 / C,其中60:40是每种金属的摩尔比,在最佳反应条件(360°C和WHSV = 1 h -1)下,油酸转化率为90.5%,对C 17和C的选择性为80.5%。C 18实现了石蜡烃。此外,还进行了基于机理的综合动力学建模-考虑了电费率定律,L–H和E–R模型。使用Levenberg-Marquardt算法通过非线性回归在速率数据拟合中研究了从这三种动力学模型得出的动力学表达式。根据统计判别标准,以表面反应为速率控制步骤,通过L–H速率方程可以最佳拟合四氢化萘脱氢反应的实验数据。相反,假设油酸与解离性H 2单点吸附,油酸脱氧反应的动力学数据与L–H速率方程密切相关。吸附。发现整个反应的限速步骤是油酸的氢化,其活化能为75.0±5.1kJ mol -1,而四氢化萘的脱氢具有较低的活化能为66.4±2.7kJ mol -1。


























 京公网安备 11010802027423号
京公网安备 11010802027423号