当前位置:
X-MOL 学术
›
Energy Environ. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Simultaneous interfacial chemistry and inner Helmholtz plane regulation for superior alkaline hydrogen evolution
Energy & Environmental Science ( IF 32.5 ) Pub Date : 2020-07-21 , DOI: 10.1039/d0ee02020f Bao Zhang 1, 2, 3, 4 , Lishang Zhang 1, 2, 3, 4 , Qiuyang Tan 4, 5, 6, 7, 8 , Jinsong Wang 1, 2, 3, 4 , Jia Liu 1, 2, 3, 4 , Houzhao Wan 4, 5, 6, 7, 8 , Ling Miao 1, 2, 3, 4 , Jianjun Jiang 1, 2, 3, 4
Energy & Environmental Science ( IF 32.5 ) Pub Date : 2020-07-21 , DOI: 10.1039/d0ee02020f Bao Zhang 1, 2, 3, 4 , Lishang Zhang 1, 2, 3, 4 , Qiuyang Tan 4, 5, 6, 7, 8 , Jinsong Wang 1, 2, 3, 4 , Jia Liu 1, 2, 3, 4 , Houzhao Wan 4, 5, 6, 7, 8 , Ling Miao 1, 2, 3, 4 , Jianjun Jiang 1, 2, 3, 4
Affiliation
|
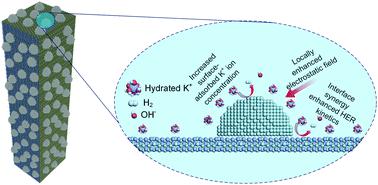
Developing highly efficient and durable alkaline hydrogen evolution reaction (HER) electrocatalysts composed of earth-abundant materials is crucial for large-scale electrochemical hydrogen production. Herein, interfacial chemistry engineering is employed to decouple the restriction of both water discharge and hydrogen adsorption free energy. And low valence state Niδ+ (δ < 1) is proposed as an efficient water-dissociation promoter based on our dual-descriptor method. Moreover, metallic and intrinsic HER-active Ni is introduced and a highly conductive edge-enriched Ni0.2Mo0.8N/Ni hybrid electrocatalyst is constructed. DFT calculations and microkinetics analysis demonstrate that the Ni site and Ni0.2Mo0.8N site have favorable hydroxyl and hydrogen species adsorption energetics, respectively, which can cooperate synergistically towards alkaline hydrogen evolution. The resulting electrocatalyst shows excellent HER electrocatalytic performance with an overpotential of about 70 mV at 300 mA cm−2 and a Tafel slope of 33 mV dec−1. It also offers outstanding operational stability at large current densities up to 200 mA cm−2. Further theoretical calculations suggest a tip-enhanced-like local electric field around the topmost Ni nanoparticles, leading to increased K-ion concentration in the inner Helmholtz plane. The HER kinetics and surface reactant density can be simultaneously improved by this hierarchical Ni0.2Mo0.8N/Ni electrocatalyst. This study might open up new avenues of reasonable design of hierarchical structures for superior electrocatalysts.
中文翻译:

同时进行界面化学反应和内亥姆霍兹平面调节,以实现出色的碱性氢释放
开发由大量地球物质组成的高效耐用的碱性氢析出反应(HER)电催化剂对于大规模电化学制氢至关重要。在本文中,采用界面化学工程来解除对水排放和氢吸附自由能的限制。基于双描述方法,提出了低价态的Niδ +(δ <1)作为高效水离解促进剂。此外,引入了金属和固有的HER-活性Ni,并构建了高导电性的边缘富集Ni 0.2 Mo 0.8 N / Ni杂化电催化剂。DFT计算和微动力学分析表明,Ni位和Ni 0.2Mo 0.8 N位点分别具有良好的羟基和氢物种吸附能,它们可以协同作用促进碱性氢的释放。所得的电催化剂显示出优异的HER电催化性能,在300mA cm -2下的过电势为约70mV,并且Tafel斜率为33mV dec -1。在高达200 mA cm -2的大电流密度下,它还具有出色的操作稳定性。进一步的理论计算表明,在最顶部的Ni纳米粒子周围会出现类似尖端的局部电场,从而导致内亥姆霍兹平面内的K离子浓度增加。Ni 0.2可以同时改善HER动力学和表面反应物密度Mo 0.8 N / Ni电催化剂。这项研究可能会为高级电催化剂的分层结构的合理设计开辟新途径。
更新日期:2020-09-16
中文翻译:

同时进行界面化学反应和内亥姆霍兹平面调节,以实现出色的碱性氢释放
开发由大量地球物质组成的高效耐用的碱性氢析出反应(HER)电催化剂对于大规模电化学制氢至关重要。在本文中,采用界面化学工程来解除对水排放和氢吸附自由能的限制。基于双描述方法,提出了低价态的Niδ +(δ <1)作为高效水离解促进剂。此外,引入了金属和固有的HER-活性Ni,并构建了高导电性的边缘富集Ni 0.2 Mo 0.8 N / Ni杂化电催化剂。DFT计算和微动力学分析表明,Ni位和Ni 0.2Mo 0.8 N位点分别具有良好的羟基和氢物种吸附能,它们可以协同作用促进碱性氢的释放。所得的电催化剂显示出优异的HER电催化性能,在300mA cm -2下的过电势为约70mV,并且Tafel斜率为33mV dec -1。在高达200 mA cm -2的大电流密度下,它还具有出色的操作稳定性。进一步的理论计算表明,在最顶部的Ni纳米粒子周围会出现类似尖端的局部电场,从而导致内亥姆霍兹平面内的K离子浓度增加。Ni 0.2可以同时改善HER动力学和表面反应物密度Mo 0.8 N / Ni电催化剂。这项研究可能会为高级电催化剂的分层结构的合理设计开辟新途径。

























 京公网安备 11010802027423号
京公网安备 11010802027423号