当前位置:
X-MOL 学术
›
Adv. Synth. Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
One‐Pot Tandem Protocol for the Synthesis of 1,3‐Bis(β‐aminoacrylate)‐Substituted 2‐Mercaptoimidazole Scaffolds
Advanced Synthesis & Catalysis ( IF 5.4 ) Pub Date : 2020-07-20 , DOI: 10.1002/adsc.202000789 Jian Luo 1 , Guo‐Shu Chen 1 , Shu‐Jie Chen 1 , Zhao‐Dong Li 2 , Yu‐Lei Zhao 3 , Yun‐Lin Liu 1
Advanced Synthesis & Catalysis ( IF 5.4 ) Pub Date : 2020-07-20 , DOI: 10.1002/adsc.202000789 Jian Luo 1 , Guo‐Shu Chen 1 , Shu‐Jie Chen 1 , Zhao‐Dong Li 2 , Yu‐Lei Zhao 3 , Yun‐Lin Liu 1
Affiliation
|
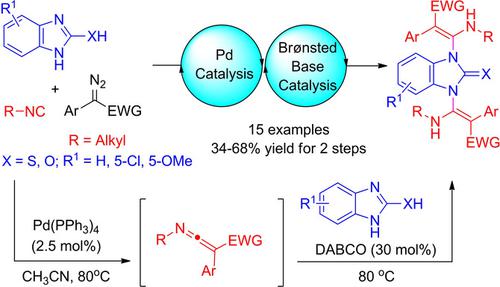
We have developed a palladium‐catalyzed cross‐coupling reaction of isocyanides with α‐diazoacetates to form active ketenimines and following a 1,4‐diazabicyclo[2.2.2]octane (DABCO) catalyzed aza‐Mannich type reaction with various 2‐mercaptoimidazoles for the synthesis of 1,3‐bis(β‐aminoacrylate)‐substituted 2‐mercaptoimidazole derivatives with structural diversity. In addition, the use of 1H‐benzo[d]imidazol‐2‐ol as the reaction partner also allowed the formation of 1,3‐bis(β‐aminoacrylate)‐substituted 2‐benzimidazolinone derivatives with moderate yields (34–52%). In the aza‐Mannich reaction, the regioselective N‐attack rather than S‐attack or O‐attack at the electrophilic central carbon of ketenimines was observed. This tandem sequence is efficient since two C=C and two C−N bonds are consecutively created in one‐pot.
中文翻译:

一锅串联方案合成1,3-双(β-氨基丙烯酸酯)取代的2-巯基咪唑支架
我们开发了钯催化的异氰酸酯与α-重氮乙酸酯的交叉偶联反应,以形成活性酮亚胺,然后在1,4-二氮杂双环[2.2.2]辛烷(DABCO)催化下的氮杂-曼尼希型反应与各种2-巯基咪唑反应制得1,3-双(β-氨基丙烯酸酯)取代的2-巯基咪唑衍生物的合成,具有结构多样性。此外,使用1 H-苯并[ d ]咪唑-2-醇作为反应伙伴还可以形成1,3-双(β-氨基丙烯酸酯)取代的2-苯并咪唑啉酮衍生物,产率适中(34-52 %)。在aza-Mannich反应中,区域选择性N攻击而非S攻击或O观察到了酮亚胺的亲电子中心碳的攻击。此串联序列是有效的,因为在一个锅中连续创建了两个C = C和两个CN键。
更新日期:2020-09-05
中文翻译:

一锅串联方案合成1,3-双(β-氨基丙烯酸酯)取代的2-巯基咪唑支架
我们开发了钯催化的异氰酸酯与α-重氮乙酸酯的交叉偶联反应,以形成活性酮亚胺,然后在1,4-二氮杂双环[2.2.2]辛烷(DABCO)催化下的氮杂-曼尼希型反应与各种2-巯基咪唑反应制得1,3-双(β-氨基丙烯酸酯)取代的2-巯基咪唑衍生物的合成,具有结构多样性。此外,使用1 H-苯并[ d ]咪唑-2-醇作为反应伙伴还可以形成1,3-双(β-氨基丙烯酸酯)取代的2-苯并咪唑啉酮衍生物,产率适中(34-52 %)。在aza-Mannich反应中,区域选择性N攻击而非S攻击或O观察到了酮亚胺的亲电子中心碳的攻击。此串联序列是有效的,因为在一个锅中连续创建了两个C = C和两个CN键。


























 京公网安备 11010802027423号
京公网安备 11010802027423号